Haryana State Board HBSE 9th Class Science Solutions Chapter 4 Structure of the Atom Textbook Exercise Questions and Answers.
Haryana Board 9th Class Science Solutions Chapter 4 Structure of the Atom
HBSE 9th Class Science Structure of the Atom Intext Questions and Answers
Questions from Sub-section 4.1
Question 1.
What are canal rays?
Answer:
E. Goldstein in 1886 discovered the positively charged fluorescent radiations, which were renamed as canal rays.
Question 2.
If an atom contains one electron and one proton, will it carry any charge or not?
Answer:
If an atom contains one electron and one proton, it will possess no charge on it. Because proton and electron mutually balance the charges.
Questions from Sub-section 4.2
Question 1.
On the basis of Thomson’s model of an atom, explain how the atom is neutral as a w hole.
Answer:
According to Thomson’s model an atom is made up of positively charged sphere and electrons get embedded into it. Thus due to uniformity in the negative and positive magnitude, an atom as a whole is electrical neutral.
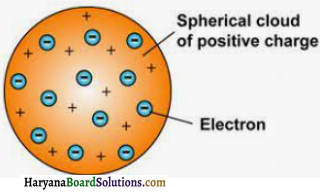
Question 2.
On the basis of Rutherford’s model of an atom, which sub-atomic particle is present in the nucleus of an atom ?
Answer:
According to Rutherford’s model of an atom, the nucleus of an atom consists of proton sub-atomic charged particle, since it deflects the a (alpha) particle.

Question 3.
Draw a sketch of Bohr’s model of an atom with three shells.
Answer:
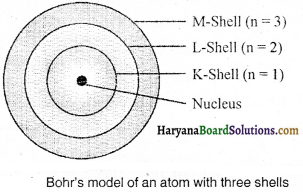
Question 4.
What do you think would be the observation if the a-particle scattering experiment is carried out using a foil of a metal other than gold ?
Answer:
Yes, the a-particie scattering experiment will be possible with any metal foil other than gold foil.
Questions from Subsection 4.2
Question 1.
Name the three sub-atomic particles of an atom.
Answer:
The three sub-atomic particles of an atom are electron, proton and neutron.
Question 2.
Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have ?
Answer:
Atomic mass of helium atom = 4u
Protons present in the nucleus of helium atom = 2u
Neutrons present in the nucleus of helium atom= Atomic mass – proton = 4 – 2 = 2

Questions from Subsection 4.3
Question 1.
Write the distribution of electrons in carbon and sodium atoms.
Answer:
(i) Carbon:
Mass number = 12
Atomic number = 6
Number of protons = 6
Number of electrons = 6
Number of neutrons = 12 – 6 = 6
Electron distribution = K = 2, L = 4
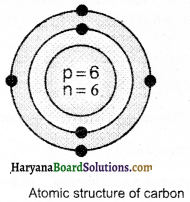
(ii) Sodium:
Mass Number = 23
Atomic number = 11
Number of protons = 11
Number of electrons = 11
Number of neutrons = 23 – 11 = 12
Electron distribution = K = 2 L = 8 M = 1
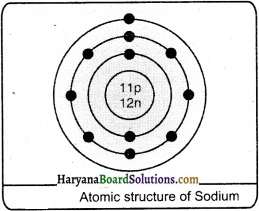
Question 2.
If K and L shells of an atom are full, then what would be the total number of electrons in the atom ?
Answer:
Shell K = 2 electrons
Shell L = 8 electrons
Total electrons in the atom = 2 + 8 = 10 electrons
Questions from Sub-section 4.4
Question 1.
How will you find the valency of chlorine, sulphur and magnesium ?
Answer:
(1) The atomic number of chlorine is 17, therefore, its electron distribution will be as under:
K = 2 electrons
L = 8 electrons
M = 7 electrons
Therefore, to complete its octet, chlorine needs (8 – 7) = 1 electron.
Hence, valency of chlorine is 1.

(2) Atomic number of sulphur is 16, therefore, its electron distribution will be as under:
K = 2 electrons
L = 8 electrons M = 6 electrons
Therefore, to complete its octet, sulphur needs (8 – 6) = 2 electrons.
Hence, valency of sulphur is 2.
(3) Atomic number of magnesium is 12.
Therefore, its electron distribution will be as under:
K = 2 electrons .
L = 8 electrons
M = 2 electrons
Therefore, to complete its valence octet, it is easy in the case of magnesium to quit 2 electrons. Hence, the valency of magnesium is 2.
Questions from Subsection 4.5
Question 1.
If the number of electrons in an atom is 8 and the number of protons is also 8, then (i) what is the atomic number of the atom? and (ii) what is the charge on the atom?
Answer:
Number of electrons in the atom = 8
Number of protons in atom = Number of electrons = 8
(i) Atomic number = Number of electrons = Number of Protons = 8
(ii) Electron distribution = K = 2, L = 6 .
To fulfil the outermost shell of atom 2 electrons are required. Therefore, the charge is -2.
Question 2.
With the help of Table 4.1. find out the mass number of oxygen and sulphur atom. Answer: According to the table,
(1) Atomic number of oxygen = 8 Number of protons in oxygen = 8
Number of neutrons in oxygen = 8
Mass number = Number of protons + Number of neutrons = 8 + 8 = 16
(2) Atomic Number of sulphur = 16 Number of protons in sulphur = ? 16
Number of neutrons in sulphur =16
Mass number = Number of protons + Number of neutrons = 16 + 16 = 32
Questions from Sub-section 4.6
Question 1.
For the symbols H, D and T tabulate three sub-atomic particles found in each of them.
Answer:
(1) Symbol H is the sign for Protium i.e. 1H1
∴ Atomic number = 1
Mass number = 1
Number of electrons = 1
Number of protons = 1
Number of neutrons = 1-1=0
(2) Symbol D is the sign for Deuterium i.e. 1H2
Atomic number = 1
Mass number = 2
Number of electrons = 1
Number of protons = 1
Number of neutrons = 2 – 1 = 1

(3) Symbol T is the sign for Tritium i.e. 1H3
Atomic number = 1
Mass number = 3
Number of electrons = 1
Number of protons = 1
Number of neutrons = 3 – 1 = 2
Question 2.
Write the electronic configuration of any one pair of isotopes and isobars.
Answer:
(i) Electronic configuration of the pair of isotope chlorine 17Cl135 and 17Cl137 will be as follows:

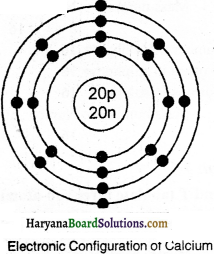
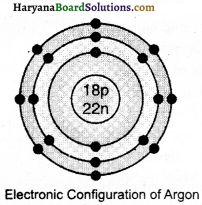
(ii) Electronic configuration of the pair of isobars calcium and argon will be as under:
(l) Calcium 20Ca40
e = 20
P = 20
N = 40 – 20 – 20
(2) Argon 18Ar40
e = 18
P = 18
N = 40 – 18 = 22
HBSE 9th Class Science Structure of the Atom Textbook Questions and Answers
Question 1.
Compare the properties of electrons, protons and neutrons.
Answer:
Properties of electrons, protons and neutrons can be compared as below:
(i) Charge: Electron is negatively charged and has an absolute charge of 1.602 x 10-19 coulomb whereas proton is positively charged and has an absolute charge of 1.602 x 10-19 coulomb. On the other hand, neutron is a neutral particle carrying no charge.
(ii) Mass: Electron has an absolute mass of 9.11 x 10-31 kg which is equal to 1/1840 amu while proton
has an absolute mass of 1.672 x 10-27kg which is equal to 1 amu. On the other hand, neutron has an absolute mass of 1.675 x 10-27kg, i.e., neutron is slightly heavier than proton.

(iii) Location: Electrons are located outside the nucleus while protons and neutrons are located inside the nucleus.
(iv) Symbol: Electron is represented as -1e0or e– proton is represented as 1p1 or p+ and neutron is represented as 0n1 or n.
Question 2.
What are the limitations of J.J. Thomson’s model of the atom?
Answer:
Although Thomson’s model of the atom was able to explain the electrical neutrality of the atom but it failed to explain the results of experiments carried out by other scientists. For example, this model could not explain the results of scattering experiments carried out by Rutherford and was, therefore, rejected in favour of Rutherford’s model of the atom.
Question 3.
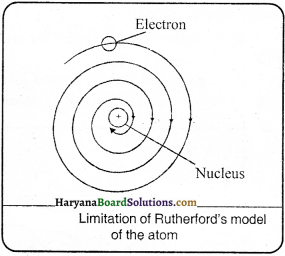
What are the limitations of Rutherford’s model of the atom?
Answer:
It was pointed by Neils Bohr that Rutherford’s atom should be highly unstable. He argued that if an electron (charged particle) moves around the nucleus in an orbit, it should be subjected to acceleration due to continuous change in its direction of motion. Therefore, the electrons should continuously emit radiations and lose energy.
Consequently, the orbit should become smaller and smaller and ultimately the electron should fall into the nucleus. In other words, the atom should collapse. Since the atoms do not collapse, therefore, there must be something wrong with Rutherford’s model of atom. Another serious drawback of Rutherford’s model of an atom is that it says nothing about the electronic structure of the atom, i.e., how electrons are distributed around the nucleus and what are the energies of these electrons.
Question 4.
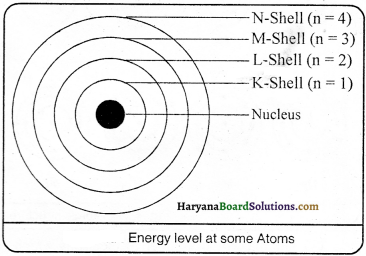
Describe Bohr’s model of the atom.
Answer:
According to Neils Bohr’s model of the atom
(1) Electrons revolve or move in definite orbits which are known as discrete orbits of electrons.
(2) When electrons revolve in discrete orbits, they do not radiate energy. These orbits (or shells) are called energy levels. Energy levels in an atom are shown in the figure.
Question 5.
Compare all the proposed models of an atom given in this chapter.
Answer:
A comparison of all the proposed models of an atom given in this chapter is as follows:
1. According to Thomson’s model of an atom:
(1) An atom consists of a positively charged sphere and the electrons are embedded in it.
(2) The negative and positive charges are equal in magnitude. So, atoms are electrically neutral.
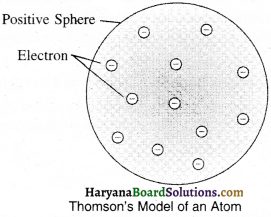
2. According to Rutherford’s Model of an atom:
(1) The centre in the atom is positively charged. About all the mass of an atom resides in the nucleus.
(2) The electrons revolve around the nucleus in some definite orbits.
(3) The size of the nucleus is very small as compared to the size of the atom.

3. According to Bohr’s model of an atom:
(1) The electrons can revolve only in certain definite orbits which are known as discrete orbits of electrons.
(2) When electrons revolve in discrete orbits, then they do not radiate energy. These orbits are called energy levels.
Question 6.
Summarise the rules for the writing of distribution of electrons in various shells for the first eighteen elements.
Answer:
Bohr and Bury proposed identical schemes regarding the arrangement of electrons in various orbits. The main rules of the Bohr-Bury scheme are:
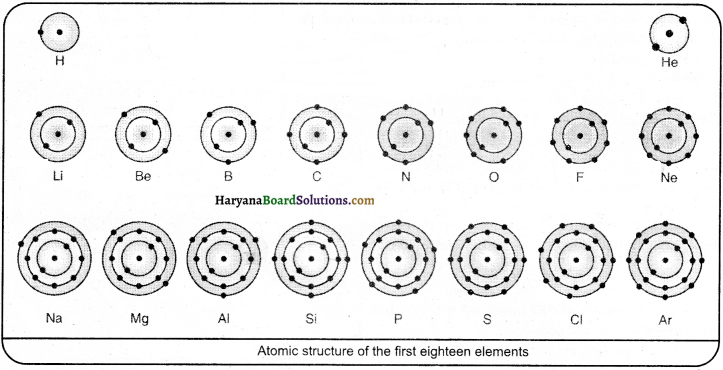
(1) The maximum number of electrons which can be accommodated in any orbit or shell is equal to 2r? where n is the number of orbit or shells.
(2) The maximum capacity of the outermost shell is of 8 electrons and that of the penultimate shell (next to the outermost) is of 18 electrons.
(3) It is not necessary that a shell should be completed to its maximum capacity before another starts. In fact, a new shell always starts when the outermost shell attains 8 electrons.
(4) The outermost shell cannot have more than 2 electrons and the penultimate shell cannot have more than 9 electrons unless the next innermost shell has received the maximum number of electrons as required by rule (i).
Question 7.
Define valency by taking examples of silicon and oxygen.
Answer:
Valency of an element is the combining capacity of the element and is equal to the number of electrons that take part in chemical reaction. The electronic configuration of silicon is :
K L M
2 8 4
Since it has 4 electrons in its valence shell, therefore,
Valency of silicon = 8 – Number of valence electrons = 8 – 4 = 4
The electronic configuration of oxygen is:
K L
2 6
Since it has 6 electrons in its valence shell, therefore,
Valency of oxygen = 8 – Number of valance electrons = 8 – 6 = 2.

Question 8.
Explain with examples
(i) Atomic number
(ii) Mass number
(iii) Isotopes
(iv) Isobars. Give any two uses of isotopes.
Answer:
(i) Atomic Number:
The total number of protons present in the nucleus of an atom of an element is known as atomic number (Z). for example, the atomic number of oxygen is 8 and that of carbon is 6.
(ii) Mass Number:
The total number of protons and neutrons present in an atom of an element is known as its Mass number (A). For example, the mass number of oxygen and carbon is 16u and 12u respectively.
(iii) Isotopes:
Atoms of the same element having same atomic number but different mass numbers are known as isotopes. For example, Protium (1H1), deueterium (1H2), and tritium (1H3) are three isotopes of hydrogen and 6C12 and 6C14 are two isotopes of carbon.
(iv) Isobars: Atoms of different elements which have same mass number but different atomic numbers are known as isobars. For example, calcium (20Ca40) and argon (18Ar40) are isobars.
Uses of Isotopes:
(1) Isotope of uranium is used as a fuel in nuclear reactors.
(2) Isopote of cobalt is used in the treatment of cancer.
Question 9.
Na+ has completely filled K and L shells. Explain.
Answer:
The atomic number ofNa (sodium) is 11, so an atom of Na contains 11 electrons. The arrangement of electrons in Na atom will be:
K L M
2 8 1
Now, Na+ ion is formed by loss of 1 valence shell electron, therefore, the remaining 10 electrons are arranged as:
K L
2 8
According to Bohr and Bury rule (2n2 formula), the K and L shells can accommodate 2 and 8 electrons respectively. This explains that the K and L shells in Na+ ions are completely filled.

Question 10.
If the bromine atom is available in the form of, say, two isotopes, 3579Br(49.7%) and 3581Br (50.3%), calculate the average atomic mass of the bromine atom.
Answer:
Average atomic mass of bromine atom
\(\left(79 \times \frac{49.7}{100}+81 \times \frac{50.3}{100}\right)\)
\(\left(\frac{79 \times 497}{1000}+\frac{81 \times 503}{1000}\right)\)
\(\left(\frac{39263}{1000}+\frac{40743}{1000}\right)\) = 39.263 + 40.743 = 80.006 = 80u
Question 11.
The average atomic mass of a sample of element X is 16.2 u. What are the percentages 16 18 of isotopes 816X and 188 X in the sample?
Answer:
Let the percentage of isotope 168X be x. Then the percentage of 188X is (100 – x).
Now,
Average atomic mass = \(\frac{16 x+18(100-x)}{100}\)
or 16.2 = \(\frac{16 x+18(100-x)}{100}\) or 2x = 180 or x = 90%
Therefore, percentage of 816X = 90% and percentage of 818X = 100 – 90 = 10%
Question 12.
If Z = 3, what would be the valency of the element? Also, name the element.
Answer:
If the atomic number is 3, then the arrangement of electrons in an atom of the element will be:
K L
2 1
As the valence shell contains one electron only, so valency will be one. The element with atomic number 3 is Lithium.
Question 13.
Composition of the nuclei of two atomic species X and Y are given as under:
X Y
Protons 6 6
Neutrons 6 8
Give the mass numbers of X and Y. What is the relation between the two species?
Answer:
Since Mass number = Number of protons + Number of neutrons,
Therefore, the Mass numbers of X and Y are 12 and 14 respectively.
Again, Atomic number = Number of protons = Number of electrons.
Therefore, the atomic numbers of both X and Y are 6 each.
As X and Y have the same atomic number but different mass numbers, therefore, these are isotopes of each other and are represented as 612X and 614Y.

Question 14.
For the following statements write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about \(\frac {1}{2000}\) times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Answer:
(a) F (b) F (c) T (d) F.
Put a tick (√) against the correct choice and cross (✗) against the wrong choice in questions 15, 16 and 17.
Question 15.
Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic Nucleus
(b) Electron
(c) Proton
(d) Neutron.
Answer:
(a) √ (b) ✗ (c) ✗ (d) ✗.
Question 16.
Isotopes of an element have (a) the same physical properties (b) different chemical properties (c) different number of neutrons (d) different atomic numbers.
Answer:
(a) ✗ (b) ✗ (c) √ (d) ✗
Question 17.
Number of valence electrons in Cf ion are :
(a) 16
(b) 8
(c) 17
(d) 18
Answer:
(a) ✗ (b) √ (c) ✗ (d) ✗
Question 18.
Which one of the following is a correct electronic configuration of sodium ?
(«) 2, 8,
(b) 8, 2,1
(c) 2,1, 8
(d) 2, 8,1
Answer:
(a) ✗ (b) ✗ (c) ✗ (d) √
Question 19.
Complete the following table.
| Atomic Number | Mass Number | Number of Neutrons | Number of Protons | Number of Electrons | Naine of the Atomic Species Sulphur |
| 9 | – | 10 | – | – | – |
| 16 | 32 | – | – | – | sulphur |
| – | 24 | – | 12 | – | – |
| – | 2 | – | 1 | – | – |
| – | 32 | 1 | 1 | 0 | – |
Answer:
| Atomic Number | Mass Number | Number of Neutrons | Number of Protons | Number of Electrons | Naine of the Atomic Species Sulphur |
| 9 | 19 | 10 | 9 | 9 | Fluorine |
| 16 | 32 | 16 | 16 | 16 | sulphur |
| 12 | 24 | 12 | 12 | 12 | Magnesium |
| 1 | 2 | 1 | 1 | 1 | Deuterium |
| 1 | 32 | 1 | 1 | 0 | Hydrogen |
Must Read:
BHARATFORG Pivot Point Calculator
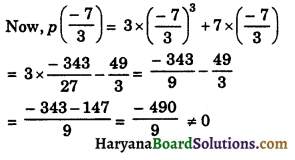


 =\(\frac {x}{30}\) h
=\(\frac {x}{30}\) h