Haryana State Board HBSE 9th Class Science Important Questions Chapter 1 Matter in Our Surroundings Important Questions and Answers.
Haryana Board 9th Class Science Important Questions Chapter 1 Matter in Our Surroundings
Very Short-Answer Type Questions
Question 1.
What is meant by matter?
Answer:
Matter are substances which have mass and which occupy space (volume).
Question 2.
What is every object in the world made up of?
Answer:
Every object in the world is made up of matter.
Question 3.
How many basic elements have the ancient philosophers in India classified matter into ?
Answer:
The ancient philosophers in India classified matter into five basic elements.
Question 4.
What is meant by ‘Panch Tatvas’?
Answer:
Five basic elements-air, earth, fire, water and sky are called as ‘Panch Tatvas’.
Question 5.
How many basic elements did the Greek philosophers agree that matter possesses?
Answer:
The greek philosophers agreed that matter is composed of four basic elements i.e., earth, fire, air and water.
![]()
Question 6.
What is the basis of the classification of matter in modern science?
Answer:
In modem science, the basis of classification of the matter is physical properties and chemical nature.
Question 7.
Write down four examples of solid materials.
Answer:
Stone, wood, iron and ice.
Question 8.
Write down four examples of liquid materials.
Answer:
Water, kerosene, spirit and petrol.
Question 9.
Write down four examples of gaseous materials.
Answer:
Oxygen, nitrogen, carbon dioxide and water vapour.
![]()
Question 10.
On the basis of physical state how many types of matter are there?
Answer:
On the basis of physical state, matter is available in three states
(i) solid
(ii) liquid
(iii) gas.
Question 11.
How many types of matter are there on the basis of chemical formation?
Answer:
On the basis of chemical formation matter is of three types –
(i) element
(ii) compound
(iii) mixture.
Question 12.
Write the name of the solid state of water.
Answer:
The solid-state of water is ice.
Question 13.
Which are the three different states of water?
Answer:
The three different states of water are –
(i) ice (solid)
(ii) liquid (water)
(iii) Eteam (gaseous).
Question 14.
Write down the physical properties of solids.
Answer:
Solids are completely incompressible, they have definite size, shape and volume.
Question 15.
What are the physical properties of liquids?
Answer:
Liquids are comparatively incompressible fluids. They have a definite volume, but the shape and size is indefinite.
![]()
Question 16.
What are the physical properties of gas?
Answer:
Gas is an excessively compressible fluid. The given quantity of gas will fill up in containers of any size and shape. .
Question 17.
What property of gas enables it to inflate maximum air in the type?
Answer:
Due to the property of compressibility of gas.
Question 18.
What is meant by CNG?
Answer:
CNG means Compressed Natural Gas.
Question 19.
What do you mean by intermolecular force?
Answer:
The forces of cohesion applicable in between the particles of substances (molecules or atoms) is called intermolecular force.
Question 20.
Which has the maximum intermolecular force: solid, liquid or gas?
Answer:
Solid has the maximum intermolecular force.
Question 21.
Which has the minimum intermolecular force: solid, liquid or gas?
Answer:
Gas has the minimum intermolecular force.
Question 22.
Why do solids have definite shapes?
Answer:
Due to random intermolecular force solids have definite shapes.
Question 23.
Do the molecules of matter have hollow spaces in between them?
Answer:
Yes, the molecules of matter have fair hollow space in between them.
Question 24.
What is meant by diffusion?
Answer:
Self-intermixing of molecules of two different substances into one another is called diffusion.
![]()
Question 25.
Why does the aroma of a burning incense stick spread at a far distance?
Answer:
Due to diffusion, the aroma of a burning incense stick spreads at a far distance.
Question 26.
What characteristic of gases enables us to find the leakage of LPG?
Answer:
Due to the diffusion characteristics of gases leakage of LPG is found out.
Question 27.
How does the flavour of ether and cooking reach us?
Answer:
Gases diffuse in air very quickly. Due to this very property, the flavour of ether and cooking reaches us.
Question 28.
How is the purity of honey tested?
Answer:
If on pouring down a drop of honey in a glass of water the drop of honey creeps down in the form of a coloured streak, then the honey is supposed to be pure otherwise the honey is supposed to be impure.
Question 29.
What effect does the temperature leave on the speed of molecules of matter?
Answer:
With the increase in temperature, the speed of the molecules accelerates.
Question 30.
Give an example of such a solid which changes its shape on stretching it.
Answer:
Rubber band.
Question 31.
Give an example of a compressible solid.
Answer:
The sponge is a compressible solid.
Question 32.
Which one is related to flowing, out of solid or liquid?
Answer:
The liquid is related to flow.
![]()
Question 33.
Write down names of two miscible gases.
Answer:
Oxygen and carbon dioxide are miscible gases.
Question 34.
Where do the aquatic animals receive oxygen from to breathe?
Answer:
The aquatic animals make use of oxygen dissolved in water to breathe.
Question 35.
Which gas does the balloon-seller fill into the balloon ?
Answer:
The balloon-seller fills hydrogen gas in the balloon.
Question 36.
Which gas is used in houses to cook?
Answer:
Liquefied Petroleum Gas (LPG).
Question 37.
Which gas cylinders are used in hospitals for artificial respiration?
Answer:
Oxygen gas cylinders are used in hospitals for artificial respiration.
Question 38.
In solids, liquids and gases which one has the maximum compressibility property?
Answer:
Gases have the maximum compressibility.
Question 39.
What is called the irregular speed of dust particles in air?
Answer:
The irregular speed of dust particles in air is called Brownian speed.
Question 40.
What happens when ice is heated up?
Answer:
When ice is heated up, it converts into (liquid) water.
Question 41.
What is formed when liquid (water) is heated up?
Answer:
On heating up liquid (water) vapours (steam) is formed.
![]()
Question 42.
Out of solid, liquid or gas in which case molecules are totally free to move?
Answer:
In gas the molecules are totally free to move.
Question 43.
What is the use of stirring a liquid while heating it up?
Answer:
While heating up if the liquid is stirred constantly it will get heated equally overall.
Question 44.
Write down name of the device to measure temperature.
Answer:
Thermometer.
Question 45.
What is meant by melting point?
Answer:
That fixed temperature at which a solid becomes liquid on melting is called melting point.
Question 46.
What is SI unit of temperature?
Answer:
SI unit of temperature is ‘Kelvin’ (K).
Question 47.
What is done to convert Kelvin temperature into Celsius temperature?
Answer:
In order to convert Kelvin temperature into Celsius temperature 273 is to be subtracted from the given temperature.
Question 48.
Convert 373 K into Celsius temperature.
Answer:
373 K = (373 – 273)° C = 100° C
Question 49.
How is celsius temperature converted into Kelvin temperature?
Answer:
To convert celsius temperature into Kelvin temperature 273 is added to the given temperature.
Question 50.
Convert 0° C into Kelvin temperature.
Answer:
0° C = (0 + 273) K = 273 K
Question 51.
What is the melting point of ice?
Answer:
The melting point of ice is 273.16 K.
Question 52.
What is meant by melting?
Answer:
The process of melting, i.e., conversion of a solid into a liquid state is called melting.
Question 53.
What is meant by the dormant heat energy of melting?
Answer:
At atmospheric pressure, the heat energy that is required to convert 1 kg of solid at its melting point into a liquid state, is called dormant heat energy of melting.
![]()
Question 54.
Which of the two, water or ice’s molecules will possess more energy at 0° C?
Answer:
At 0° C, the molecules of water will possess more energy than ice.
Question 55.
What is meant by boiling point ?
Answer:
At atmospheric pressure, the temperature at which the liquid starts boiling is called boiling point.
Question 56.
What is the boiling point of water?
Answer:
The boiling point of water is 373 K.
Question 57.
What is meant by in exposed evaporation or dormant heat energy of evaporation?
Answer:
At atmospheric pressure, the heat energy that is required to convert 1 kg of liquid at its boiling point into water vapours, is called inexposed evaporation or dormant heat.
Question 58.
Which of the two, steam or water will render more burning sensation at 373 K temperature?
Answer:
At 373 K temperature, steam will render more burning sensation than water, for its molecules possess yet more in exposed heat of evaporation.
Question 59.
What is meant by sublimation?
Answer:
Without changing into liquid state, the process of changing of solid, directly into gaseous and again in solid state is called sublimation.
Question 60.
Write names of two sublime substances.
Answer:
(i) Camphor
(ii) ammonium chloride.
Question 61.
What happens when pressure is increased on any gas?
Answer:
On increasing pressure on any gas it changes into liquid.
![]()
Question 62.
What is meant by dry ice?
Answer:
Dry carbon dioxide is called dry ice.
Question 63.
What is the SI unit of pressure?
Answer:
The SI unit of pressure is Pascal (Pa).
Question 64.
What is the atmospheric pressure on the sea surface?
Answer:
On the sea surface the atmospheric pressure used to be 1 atmosphere.
Question 65.
What is meant by ‘evaporation’?
Answer:
The process of changing of liquid into vapours below the temperature of boiling point is called evaporation.
Question 66.
Write down one advantage of evaporation in daily life.
Answer:
In daily life, wet clothes dry up because of evaporation.
Question 67.
What is the effect of the level region on evaporation?
Answer:
An increase in the level region, the evaporation rate increases.
Question 68.
What is the relation of evaporation with temperature?
Answer:
With the increase in temperature, the evaporation rate increases.
Question 69.
What is meant by humidity?
Answer:
The quantity of water vapours present in the air is called humidity.
Question 70.
What is the effect of humidity on evaporation?
Answer:
With the increase in humidity, the evaporation rate decreases.
![]()
Question 71.
Why do people sprinkle water on their roofs and in the open places after the scorching bright sunshine day comes to an end?
Answer:
Because the dormant heat of evaporation cools down the hot surface.
Question 72.
Which of the following substances will you expect to possess the strongest and the weakest intermolecular force: water, alcohol, sugar, sodium chloride, carbon dioxide?
Answer:
Sodium chloride will have the strongest and carbon dioxide will have the weakest intermolecular force.
Question 73.
Compression in gases is possible, whereas that of liquids is not. Why is it so?
Answer:
Because of too much intermolecular gap distance in gases, they can be compressed. But in liquids, due to less intermolecular gas the distance they cannot be compressed.
Short-Answer Type Questions
Question 1.
How has the classification of matter been done in ancient period in context with the modern science?
Answer:
Classification of matter:
(1) In ancient times, the ancient philosophers had the concept that matter is composed of five basic elements i.e.,
(i) air
(ii) earth
(iii) fire
(iv) water
(v) sky (all were said to be Panch Tatvas)
all the living or non-living things according to them were bom out of these five elements, whereas the Greek Philosophers are of the view that there have been only four elements in all i.e.,
(i) air
(ii) earth
(iii) fire and
(iv) water.
(2) The modem scientists on the other hand, on the bases of physical properties and chemical nature have classified matter into two kinds.
Question 2.
How is the classification of matter done according to the physical properties?
Answer:
According to physical properties matter are divided into three categories:
1. Solids: Solids are completely incompressible, of definite shape and volume; like stone, wood, iron, ice, salt, sugar, etc.
2. Liquids: Liquids are comparatively less incompressible. They have a definite volume but have an indefinite shape like water, kerosene, spirit, petrol, milk, etc.
3. Gases: Gas is an excessively compressible fluid. Its volume and shape is indefinite which means the gas of a given quantity can be filled in any container of any shape like oxygen, nitrogen, carbon dioxide, water vapours, methane, etc.
![]()
Question 3.
Write four special properties of solid matter.
Answer:
Special properties of solid matter are as follows:
(1) They can be collected in the form of piles.
(2) They have a definite volume.
(3) They can be scratched.
(4) Solid substances are hard to touch.
(5) They have definite shapes.
Question 4.
What are the general properties of liquid matter?
Answer:
The general properties of liquids are as follows:
(1) Liquids cannot be placed in piles.
(2) All liquids can flow.
(3) Liquids cannot be scratched.
(4) All liquids have definite volumes.
(5) All liquids form the shape of that very container in which they are kept that is they do not have a definite shape.
Question 5.
Which of the properties are common in all in solids, liquids and gases?
Answer:
The following properties are common in all in solids, liquids and gases:
(1) All are composed of molecules and atoms collectively.
(2) All occupy space.
(3) All have mass.
(4) All can be felt through our senses.
Question 6.
Give the general properties of gases.
Answer:
The general properties of gases are:
(1) Gases do not have definite shape and volume.
(2) Gases dissolve into air.
(3) Gases cannot be scratched.
(4) Gases can be collected only in enclosed containers.
(5) The gas spreads in the container in which it is kept, it spreads at that very place where it is released.
(6) Gases can flow.
![]()
Question 7.
Clarify your answer with suitable examples that the properties of all the liquid matter do not resemble one another.
Answer:
The following examples make it clear that the properties of all the liquid matter do not resemble with one another:
(1) Some liquids have low ignition point like petrol, but there are yet certain liquids which do not catch fire like water.
(2) Some liquids are comparatively heavier in weight like mercury. Some liquids are not too heavier like kerosene oil.
(3) Some liquids flow’ quickly like water. Some liquids flow slowly, for example, molten sugar and honey.
(4) Some liquids have the property to saturate salt, sugar, etc. in them. Some liquids cannot saturate these in them like mustard oil.
(5) Some liquids are colourless like kerosene oil, whereas some liquids are coloured like mustard oil.
(6) Some liquids evaporate earlier like spirit. Some liquids evaporate later like turpentine oil.
Question 8.
Compare the intermolecular force in molecules or atoms in solids, liquids and gases.
Answer:
In solids, molecules or atoms are densely and compactly arranged and they are tied up with one another by means of force of attraction whereas in liquids, atoms or molecules are thinly and loosely arranged. In solids, molecules or atoms oscillate about their respective positions, while in liquids, molecules or atoms can move about here and there within their fixed path. In gases, molecules or atoms are quite far away from one another and they move randomly. These molecules or atoms are free to move about within maximum space.
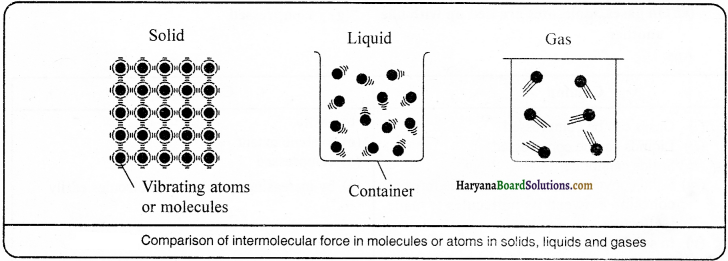
Question 9.
Clarify the reason of generating of pressure in gases.
Answer:
Since in a gaseous state, the motion of molecules is free at all. they can move about in a container, they can collide with one another and also, with the walls of the container. Due to the regular collision of them against the walls, a definite force gets produced. Consequently, pressure is generated in gases.
![]()
Question 10.
When we turn ice into water by heating process and further convert water into steam by heating it up, then what differences do we observe in the compactness of the molecules of water ?
Answer:
Ice is a solid substance and its molecules are associated with one another due to the force of cohesion. On heating ice, its molecules get active and start moving on receiving energy with the result, their force of cohesion gets loosened and molecules start flowing that means the solid ice forms the shape of liquid. When water is heated up the random speed of molecules speeds up very much and they generally leave apart from one another to a great extent and become free to move about that is, their intermolecular force weakens too much, such a state of water is said to be steam (gas).
Question 11.
Fill up the blanks choosing the suitable word or words from the list given below: too much, definite, another, very closely, freely, move, container, force, attraction, shape
(i) In gaseous state the molecules move.
(ii) In liquid state molecules can in it.
(iii) Molecules of a solid are packed and can move at a speed.
(iv) The containers in which the liquids are kept, they form the of them.
(v) The gap distance between the molecules of a gas is
(vi) In solids the molecules are joined together with strong forces of.
Answer:
(i) freely
(ii) move
(iii) very closely, definite
(iv) shape
(v) too much
(vi) attraction.
Question 12.
Match the statements given in column A by selecting appropriate words or phrases from column B:
| Column A | Column B |
| (i) Gases can be compressed | (a) by increasing speed of its molecules |
| (ii) Liquids can be compressed | (b) easily |
| (iii) Solids cannot be | (c) do not remain tied up with one another |
| (iv) Solid converts into liquid when the force of cohesion between its molecules is made in effective | (d) less strongly |
| (v) In solids, molecules are interwoven with one another | (e) to some extent |
| (vi) In liquids, molecules are tied up with one another | (f) strongly |
| (vii) In gases, molecules | (g) compressed |
Answer:
| Column A | Column B |
| (i) Gases can be compressed | (b) easily |
| (ii) Liquids can be compressed | (e) to some extent |
| (iii) Solids cannot be | (g) compressed |
| (iv) Solid converts into liquid when the force of cohesion between its molecules is made in effective | (a) by increasing speed of its molecules easily |
| (v) In solids, molecules are interwoven with one another | (f) strongly |
| (vi) In liquids, molecules are tied up with one another | (d) less strongly |
| (vii) In gases, molecules | (c) do not remain tied up with one another |
Question 13.
The shape of a rubber band can be changed by stretching it, but even then it is not considered as a solid, why ?
Answer:
The shape of the rubber band can be changed by stretching it, but even then it is not considered as a solid, it is because of the reason that on applying external force the rubber band changes its shape and on the removal of the external force it again retains its original shape, whereas it breaks up if the applied force is beyond a limit.
![]()
Question 14.
Sugar and salt form the shape exactly of the vessels of different shapes in which they are kept, but still they are considered as solids, why ?
Answer:
Sugar and salt form the shape of the vessels of different shapes in which they are kept, but still they are considered as solids, it is because the shape of their crystals do not change.
Question 15.
A sponge, even being a solid gets compressed, why?
Answer:
Even being a solid, a sponge gets compressed, it is because the sponge consists of small pores in it, which keep trapped air in them. When we press or compress it, the air escapes out from these pores and the sponge gets compressed.
Question 16.
In fluids, diffusion of three states (solid, liquid and gas) is possible, why?
Answer:
In fluids, diffusion of solids, liquids and gases is possible. In liquids the diffusion rate is comparatively higher, in a liquid state, the molecules of a substance move freely and in comparison to solids, the molecules of liquids occupy more vacant space.
![]()
Question 17.
The temperature remains equal when a solid undergoes the process of melting, where does the heat energy go during the process?
Answer:
During the melting process any solid (like ice), on reaching the melting point, till the solid melts as a whole, the temperature does not change. Despite providing heat to the beaker, it happens so. By bringing under control the intermolecular force of the molecules, while in changing the state of the matter this heat energy is utilised, because without indicating any increase in the temperature, this heat energy is absorbed by the solid (ice), this thing is assumed that it remains hidden in the material of the beaker, which is called latent heat. At atmospheric pressure, the amount of heat energy that is required to convert 1 kg of solid at its melting point into liquid, is called dormant heat energy of melting, at 0° centigrade (273 K) the energy of the molecules of water is more than that of the molecules of ice at a similar temperature.
Question 18.
Convert the following Celsius measures into Kelvin measuring sequence:
-273°C; -100°C; -40°C; 30°C; 2000°C
Answer:
We know that K = °C + 273
Therefore,
(i) -273°C = -273 + 273 = 0 K
(ii) -100°C = -100 + 273 = 173K
(iii) – 40°C = – 40 + 273 = 233 K
(iv) 30°C = 30 + 273 = 303 K
(v) 2000°C = 2000 + 273 = 2273
Question 19.
Air, at 82 K temperature changes into liquid and at 61K temperature, converts into solid Convert these temperatures in Celsius measuring sequence:
Answer:
We know that
(i) While keeping K = 82
K = °C + 273
82 = °C + 273
⇒ °C = 82 – 273 -191
So, the temperature of the liquefied air = 82 K = -191°C
(ii) Assuming K = 61
61 = °C + 273
⇒ °C = 61 – 273 = -212
So, the temperature of solidified air = 61 K = – 212°C
Question 20.
Why is ice, with a temperature 0°C more effective for cooling than water at 0°C ?
Answer:
We know, the dormant heat energy of melting of ice is 335 J/g. Therefore, ice with 0°C does have comparatively more heat than water at 0°C. Hence, ice with 0PC is more effective than the water with 0°C for cooling.
Question .21.
Why does our body feel cooler in the Himalayan region during winters?
Answer:
In the Himalayan region during winters, the temperature of environment drops below 0°C, whereas the normal temperature of our body used to be 37°C or 98.6° F. Just because of this drastic difference in temperature we feel more chilled in those regions during winters. Covering the body with woollen clothes the inner heat of the body gets maintained and the low temperature outside does not affect our body.
![]()
Question 22.
What is meant by evaporation and boiling ?
Answer:
Evaporation: During this process the liquid converts into water vapours before it gets boiled. Evaporation generally occurs on the outer surface of a liquid at all temperatures.
Boiling: When the pressure of any liquids surface equalises the atmospheric pressure, then the liquid starts converting into vapours. This process is called the boiling of a liquid.
Question 23.
Why do the wet clothes dry up sooner if they are spread out ?
Answer:
The speed of evaporation depends upon the surface of the wet article. When the wet clothes are spread out to dry up, the size of the wet surface area gets increased with that evaporation takes place fastly. For that reason the wet clothes dry up earlier when they are spread out and put to dry.
Question 24.
When during winters the water in the tank thaws, then how the fishes living in the tank survive ?
Answer:
During chilly winter days the water in the tank thaws at 0°C into ice, but in this process, only the outer layer of the water surface freezes and the water below to that layer does not thaw. Due to this non-uniform expansion when water is cooled down below 4°C temperature, it instead of contracting expands up to 0°C. For that reason, water thaws into lighter ice and ice start floating on the water. The fish found in such water and the other aquatic animals move to the liquid water below to ice and they survive there.
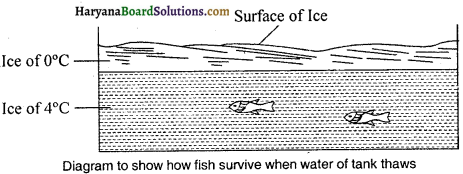
Question 25.
Where do water vapours come from in the atmosphere?
Answer:
When we put wet clothes in the sun, they dry up sooner. The water present in the fibres of the clothes on evaporating becomes a part of the atmosphere. Similarly, water evaporates in a huge quantity from lakes, rivers and oceans Air cannot sustain water vapours at a given temperature more than a fixed amount. When there is the maximum amount of water vapours in air, the air is said to be quenched.
Question 26.
Why do people sprinkle water on floor and roofs during summers ?
Answer:
People sprinkle water on floor and roofs during summer because water evaporates for which it gets heat from the floor and the roof and thereby keeps them cool. Therefore to keep floor and roofs cool, people sprinkle water on them in summer.
Essay Type Questions
Question 1.
Write down the difference in the following :
(i) heat and temperature
(ii) boiling and evaporation
Answer:
(i) Following are the differences between heat and temperature
Heat:
1. Heat is that form of energy in which we feel the sensation of hot and cool.
2. This is a form of energy.
3. It is measured in calorie or kilocalorie. SI unit is joule (J).
4. It depends upon the form, temperature and nature of a substance.
5. It is a reason.
6. It is the amount of energy in a substance.
7. It is measured by calorimeter.
Temperature:
1. This is that property of a substance which fixes the flow of heat.
2. This is a state by which we come to know the direction of flow of heat.
3. It is measured in parts1 like centigrade and kelvin.
4. It does not depend upon these things.
5. It is the effect of heat.
6. It is a physical property of a substance.
7. It is measured with thermometer.
(ii) Following are the differences between boiling and evaporation
Boiling:
1. It is a random process.
2. It exists only at a fixed temperature.
3. It exists in the entire liquid.
4. This process produces sound.
5. Coolness does not produce in it.
6. Steam is produced in it which is visible.
7. The properties of liquids, open base and the direction of wind do not affect this process.
8. In this process, bubbles are seen moving up wards.
![]()
Evaporation:
1. It is a slow process.
2. It exists at all temperatures.
3. It exists only at the bottom level of the liquid.
4. This is a mute process.
5. Coolness produces in it.
6. Water vapours are formed which get mixed into air. Water vapours are invisible.
7. This process depends upon all these things.
8. In this process, bubbles are not produced.
Question 2.
On what factors does the process of evaporation depend ?
Answer:
The process of evaporation is affected by the following factors:
(1) On being the air become dr}’, the evaporation process goes rapidly.
(2) Also, due to fast speed of wind the process of evaporation speeds up.
(3) Even when the base of the liquid is open the process of evaporation will take place randomly.
(4) With the increase in the temperature of the liquid evaporation process will be faster.
(5) If the temperature of air increases the speed of the evaporation process will be rapid.
(6) In more volatile liquids like spirit, petrol, etc. evaporation takes place quickly.
(7) Due to low pressure evaporation process accelerates.
Question 3.
Why does coolness occur with the evaporation of water ? Give some of its uses in our daily life.
Answer:
Coolness occurs due to evaporation, because when water evaporates, it needs latent heat energy of evaporation. This heat can be obtained from that substance which is in the contact of water, thus that substance becomes somewhat cool.
Uses of Evaporation in Daily Life:
(1) Water remains cool in an earthen pot, but not in a bucket, because there are pores in the pot. Water seeps out of them and it evaporates which produces coolness. Thus, water remains cool.
(2) During summer the trees bear more leaves and from the stomata of these leaves evaporation takes place, as a result coolness produces and the trees remain cool.
(3) During perspiration, fanning brings the sensation of coolness, because by fanning the sweat evaporates which produces coolness. For evaporation of sweat, heat is obtained from the body itself.
(4) On sprinkling water on the ground there is coolness for when evaporation of water takes place, then it gains heat from the earth, so the earth becomes cool.
(5) After bathing we feel cool, it is because the body is wet. The evaporation of water takes place and for evaporation, heat is obtained from the body, hence we feel cold.
Practical Work
Experiment 1:
Prove through an activity that matter are composed of molecules and molecules have vacant space or gap in between them.
Procedure:
Take a graduated measuring cylinder. Fill % part of it with water and note down the surface level. Now add some sugar to it and note down its surface level. You will notice that the surface level of water has increased somewhat.
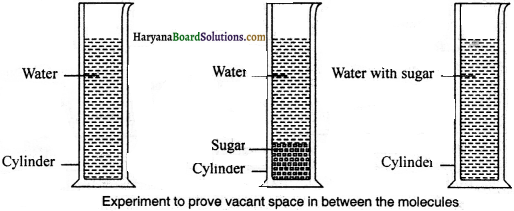
Now, stir well sugar in the water. Sugar will dissolve in water and the surface level of water will again reduce. This proves, that there is gap between molecules of water. While stirring the molecules of sugar-filled up that vacant space, consequently, the water level inside the cylinder was reduced. Since matter is composed of molecules, hence sugar got dissolved in the whole of the water.
![]()
Experiment 2:
Prove experimentally, the molecules of matter are so small in size, that we cannot even imagine.
Procedure:
Take a beaker, add 100 ml of water in it. Add two or three crystals of potassium permanganate and dissolve. Draw out approximately 10 ml of solution from the beaker, add 90 ml of pure water to it. Again draw out 10 ml of solution and add 90 ml of pure water to it. Repeat this process 5 to 8 times. You will notice, the water will still remain coloured. This experiment shows that with very small amount of crystals of potassium permanganate, the huge amount of water (1000 litres) too becomes coloured. Thus, we come to the conclusion that just one crystal of potassium permanganate will have a number of minute molecules. They are so minute that we cannot even imagine.
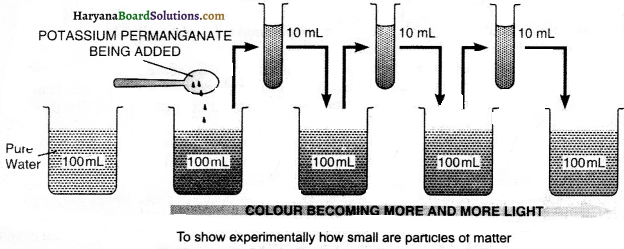
Experiment 3:
Prove experimentally that diffusion takes place more rapidly in gases than in liquids. Procedure: Make a preparation of concentrated film by adding a spoonful of potassium permanganate in 100 ml of water. Keep this solution in the beaker. Now, keep the beaker in a little bit tilted position and add 100 ml of water to it. While adding water to the beaker, be careful that the water should flow down along the wall of the beaker in such a way, so that the concentrated solution of potassium permanganate remains undisturbed. After some time you will observe two layers are formed separately which are quite clear.
![]()
Nevertheless, you will see the water gradually flowing into coloured film and the coloured film gradually flowing into water. After some time both the layers will not be seen isolated quite clearly. Note down the tune taken by both the layers to mix up with each other. Now, light a candle in one of the comers in your classroom. You stand far away at the other comer. Now, ask one of your friends to light a few incense sticks. You will very soon experience the fragrance of the incense sticks. Note down the time taken right from the time of lighting of the incense sticks, till the time taken by fragrance to reach to you. We will notice that in the latter stage total time taken was very less than that in the former stage. It proves that in gases diffusion was more rapid than in the liquids.
Experiment 4:
Illustrate experimentally, what effect does pressure have on solids, liquids and gases.
Procedure:
Take a syringe of 100 ml volume. Insert its needle (nozzle) into a rubber cork and close it up, as shown in the figure. Take away the piston and let air fill completely inside the syringe. Now, insert the piston back into the syringe carefully and make sure there is no leakage around the ends of the syringe. It would be better to apply a little bit of vaseline on the piston. Now, try to compress the air. You will notice that air will be compressed.
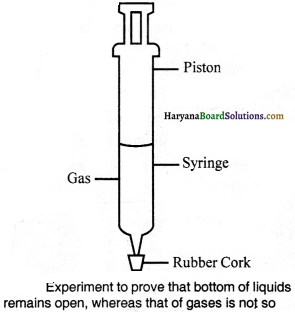
Now, fill the syringe with water and again repeat the same process. In the experiment done with water you will notice in water, compression is comparatively less than in the first one. Now, repeat the same experiment with piece of chalk in place of water, there will be no compressibility. On the bases of this experiment, we can say gases have maximum effect of pressure, liquids have lesser than that and on solids, it is almost negligible; that is, gases are the most compressible.
Experiment 5:
Experimentally show that with the change in temperature, the state of matter changes.
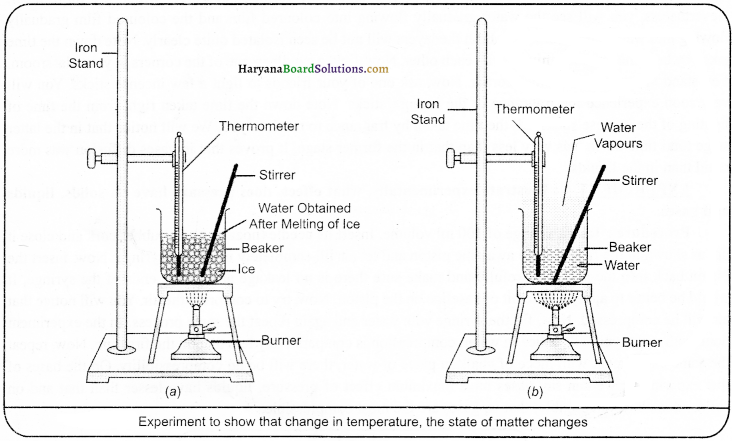
Procedure:
Take a piece of ice of 150 gm in a beaker and according to the figure hang the thermometer used in the laboratory into it such that the bulb of the thermometer should touch the ice. Start heating the beaker on mild flame. When the ice starts melting, then note down the temperature.
(1) When the whole of the ice change into water, then again note down the temperature.
(2) Note down the downfall in temperature in the change of solid into a liquid state.
(3) Now, place a glass rod into the beaker and heat it while stirring, till the water boils.
(4) Keep a constant eye on the degree of temperature in the thermometer, till most of the water converts into water vapours.
(5) Note down the drop in temperature right from the conversion of water in a liquid state into the gaseous state
Experiment 6:
Experimentally show that ammonium chloride is a sublime substance.
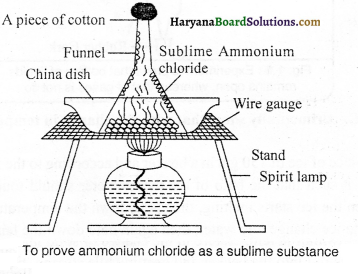
Procedure:
Take a small amount of powdered ammonium chloride in a dish. Place a funnel in an inverted position on this dish. Insert a cotton plug into the mouth of the stem of the funnel as shown in the figure. Now gently heat the dish carefully. We will notice, that ammonium chloride, without getting converted into a liquid state gets converted directly from a solid state to a gaseous state and gets deposited on the walls of the funnel. This proves that ammonium chloride is a sublime substance.
Quick Review of the Chapter
Question 1.
According to Creek philosophers, which fundamental element is not included in the matter?
(A) sky
(B) earth
(C) fire
(D) air and water
Answer:
(A) sky
Question 2.
The solution in the following is:
(A) ocean water
(B) soda water
(C) air
(D) pond water
Answer:
(B) soda water
![]()
Question 3.
On the basis of physical state, the types of matter are:
(A) elements
(B) compounds
(C) mixtures
(D) none of the above
Answer:
(D) none of the above
Question 4.
On the basis of chemical composition, the types of matter are:
(A) solids
(B) liquids
(C) gases
(D) noneoftheabove
Answer:
(D) none of the above
Question 5.
S.l. unit of weight is:
(A) Kelvin
(B) Newton
(C) Meter
(D) Pascal
Answer:
(B) Newton
Question 6.
A balloon-seller fills the balloons with:
(A) oxygen gas
(B) hydrogen gas
(C) nitrogen gas
(D) carbon gas
Answer:
(B) hydrogen gas
![]()
Question 7.
The property of compressibjIjt is highly present:
(A) in a wooden block
(B) in sponge
(C) in water
(D) in hydrogen gas
Answer:
(D) in hydrogen gas
Question 8.
Dry ice means:
(A) compressed oxygen gas
(B) compressed nitrogen gas
(C) solid carbon dioxide
(D) Acetone
Answer:
(C) solid carbon dioxide
Question 9.
To convert a kelvin temperature into a celsius temperature, what should we abstract from kelvin temperature?
(A) 80
(B) 212
(C) 273
(D) 373
Answer:
(C) 273
Question 10.
The melting point of Ice In kelvin is:
(A) 0K
(B) 80K
(C) 212K
(D) 273.16K
Answer:
(D) 273.16 K
Question 11.
Whose compression is possible?
(A) solid
(B) liquid
(C) gas
(D) none of the above
Answer:
(C) gas
![]()
Question 12.
Which cannot pile as a heap?
(A) ice
(B) salt
(C) water
(D) sugar
Answer:
(C) water
Question 13.
Which substance gets spread ¡n the complete/whole pot?
(A) ‘CC
(B) petrol
(C) kerosene
(D) methane
Answer:
(D) methane
Question 14.
Which liquid flows quickly?
(A) molasses
(B) mustard oil
(C) water
(D) honey
Answer:
(C) water
Question 15.
Which prepares liquid quickly?
(A) turpentine oil
(B) mustard oil
(C) spirit
(D) diesel
Answer:
(C) spirit
Question 16.
The normal temperature of our body Is:
(A) 370 F
(B) 37.6° F
(C) 80°F
(D) 98.6° F
Answer:
(D) 98.6° F
![]()
Question 17.
98.6°Fisequaito:
(A) 20°C
(B) 25°C
(C) 37°C
(D) 73°C
Answer:
(C) 37° C
Question 18.
Below which temperature at the cooling of water spreads instead of shrinking?
(A) 0°C
(B) 4°C
(C) 37° C
(D) none of the above
Answer:
(B) 4° C
Question 19.
Heat is measured:
(A) in centigrade
(B) in kelvin
(C) in calorie
(D) in Fahrenheit
Answer:
(C) in calorie
Question 20.
Diffusion is possible in:
(A) liquid
(B) solid
(C) gas
(D) all of the above
Answer:
(D) all of the above
Question 21.
Which substance will not cause a feeling of coolness when pouring on the palm?
(A) acetone
(B) petrol
(C) perfume
(D) honey
Answer:
(D) honey
Question 22.
The substance found in nature ¡n free state ¡n all the three states (solid, liquid, gas) is:
(A) petroleum
(B) sulphur
(C) water
(D) oxygen
Answer:
(C) water
![]()
Question 23.
Scientifically the materials by which all the things of the universe is formed, are called:
(A) wealthy
(B) resources
(C) substances
(d)) materials
Answer:
(C) substances
Question 24.
Intermixing of particles of two different substances by themselves is called:
(A) spreading
(B) diffusion
(C) compression
(D) smelling
Answer:
(B) diffusion
Question 25.
The gas which ¡s formed by compression of butane at high pressure for use in homes for making food, Is called:
(A) Liquefied Petroleum Gas
(B) Hydrogen Gas
(C) Compressed Natural Gas
(D) Natural Gas
Answer:
(A) Liquefied Petroleum Gas
Question 26.
The energy that is required to convert 1 kg of s solid st atmospheric pressure at its melting point into
liquid is called:
(A) latent heat of melting (B) latent heat of vaporisation
(C) latent heat of liquefication (D) none of these
Answer: (A) latent heat of meltmg
Question 27.
The energy that is required to convert 1 kg of liquid at atmospheric pressure at its boiling point is called:
(A) latent heat of fusion
(B) latent heat of vaporisation
(C) latent heat of Jiquefication
(D) none of the above
Answer:
(B) latent heat of vaporisation
Question 28.
Which energy Is present in particles of matter?
(A) magnetic energy
(B) static energy
(C) kinetic energy
(D) electrical energy
Answer:
(C) kinetic energy
![]()
Question 29.
What is called, that definite temperature at which a liquid starts changing into a solid?
(A) freezing point
(B) boiling point
(C) melting point
(D) ignition point
Answer:
(A) freezing point
Question 30.
The process ¡n a liquid changes into a vapour state below the temperature of its boiling point is called:
(A) meltinisation
(B) vaporisation
(C) fusionism
(D) sublimation
Answer:
(B) vaporisation
Question 31.
In which state the volume and size are not definite?
(A) solid
(B) liquid
(C) gas
(D) all of the above
Answer:
(C) gas
Question 32.
The property of hardness finds:
(A) in solids
(B) in liquids
(C) in gases
(D) in all of – liquid and gases
Answer:
(A) in solids
![]()
Question 33.
Whose compression is possible in the following?
(A) stone
(B) aluminium
(C) brick
(D) sponge
Answer:
(D) sponge
Question 34.
Lowest compression occurs:
(A) in solids
(B) in liquids
(C) in gases
(D) in gases and liquids
Answer:
(A) in solids
Question 35.
Highest compression occurs:
(A) in solids
(B) in liquids
(C) in gases
(D) in solids and liquids
Answer:
(C) in gases
Question 36.
After changing 25°C into kelvin we get:
(A) 248 K
(B) 298 K
(C) – 248 K
(D) -298 K
Answer:
(B) 298 K
Question 37.
Among water, sugar and oxygen which has the maximum intermolecular force?
(A) oxygen
(B) water
(C) sugar
(D) oxygen and water
Answer:
(C) sugar
![]()
Question 38.
What is the physical state of water at 100° C?
(A) Solid
(B) Liquid
(C) Gas
(D) Both (B) and (C)
Answer:
(C) Gas
Question 39.
Which of the following is sublimation matter?
(A) sodium chloride
(B) ammonium chloride
(C) calcium chloride
(D) all of the above
Answer:
(B) ammonium chloride