Haryana State Board HBSE 9th Class Science Important Questions Chapter 2 Is Matter Around Us Pure Important Questions and Answers.
Haryana Board 9th Class Science Important Questions Chapter 2 Is Matter Around Us Pure
Very Short-Answer Type Questions
Question 1.
What is purification?
Answer:
Separation of useful substances from unwanted and harmful substances is called as purification.
Question 2.
Write the names of any four pure substances.
Answer:
(i) Sugar
(ii) Common salt
(iii) Gold
(iv) Mercury.
Question 3.
Write the names of any four elements.
Answer:
(i) Gold
(ii) Silver
(iii) Mercury
(iv)) Sulphur.
Question 4.
Write the names of any four compounds.
Answer:
(i) Water
(if) Camphor
(iii) Pond-water
(iv) Carbon dioxide.
Question 5.
Write the names of any four mixtures.
Answer:
(i) Soil
(ii) Air
(iii) Pond-water
(iv) Rock salt.
![]()
Question 6.
Name two solute substances, soluble in water that is used in daily life.
Answer:
(i) Common salt
(ii) Sugar.
Question 7.
Write the names of two liquid mixtures.
Answer:
(i) Pond or river water
(ii) Soda water.
Question 8.
Give an example of the gaseous mixture.
Answer:
Air.
Question 9.
What is called ‘separation’?
Answer:
The separation of a substance from the other in a mixture is called ‘separation’.
![]()
Question 10.
How many components does a mixture have?
Answer:
A mixture has two or more two components.
Question 11.
Which of the useful components do we bring into use in the form of breathing from the air?
Answer:
We use oxygen from the air in the form of breathing.
Question 12.
Which property of the magnet is brought into use in magnetic separation?
Answer:
A magnet attracts iron towards it.
Question 13.
What is the principle of sedimentation?
Answer:
A combination of tiny particles forms a big and heavy molecule.
Question 14.
What is called a ‘solution’ ?
Answer:
The homogeneous mixture of two or more two substances is called a ‘solution’.
Question 15.
What is meant by ‘alloys’?
Answer:
Those homogeneous mixtures of metals which cannot be isolated from components by physical processes are called ‘alloys’.
Question 16.
Define ‘solvent’.
Answer:
That component of the solution (which has more quantity than the other) which dissolves another component in the solution is called ‘solvent’.
Question 17.
What is meant by ‘solute’?
Answer:
That component of the solution which is remained dissolved in the solvent is called as solute.
Question 18.
In the solution of sugar and water state which of them is solute and solvent?
Answer:
Solute – sugar; Solvent – water.
![]()
Question 19.
What is meant by tincture iodine?
Answer:
The solution of iodine and alcohol is called as tincture iodine.
Question 20.
Write down the solute and solvent in the tincture iodine.
Answer:
Solute – iodine; Solvent – alcohol.
Question 21.
Write down solute and solvent in soda water and coke.
Answer:
Solute – Carbon dioxide; Solvent – water.
Question 22.
Give two examples of solutions of solid within solid.
Answer:
(i) Mixture of gold and silver.
(ii) Mixture of copper and gold.
Question 23.
Give two examples of an aqueous solution.
Answer:
(i) Solution of sugar and water.
(ii) Solution of salt and water.
Question 24.
Give two examples of non-aqueous solutions.
Answer:
(i) Solution of alcohol and iodine.
(ii) Solution of sulphur and carbon dioxide.
![]()
Question 25.
Give an example of a solution of gas within the gas.
Answer:
Air is a solution of gas within the gas.
Question 26.
Which are the main components of air?
Answer:
The main components of air are nitrogen (78%) and oxygen (21%).
Question 27.
In air, which components are supposed to be solvent?
Answer:
In air, nitrogen gas is supposed to be solvent.
Question 28.
What is the diameter of the molecules of the solution?
Answer:
The diameter of the molecules of the solution is even less than 1 nm (10-9m).
Question 29.
What is meant by the unsaturated solution?
Answer:
When the quantity of the solute substance is less than saturation in a solution, then it is called an unsaturated solution.
Question 30.
What is meant by the supersaturated solution?
Answer:
If in a solution strength of solute substance is more than the strength of saturated strength, then it is called as the supersaturated solution.
Question 31.
Why is water called as a universal solution?
Answer:
Water is called a universal solution because maximum substances dissolve into it.
Question 32.
Why seawater cannot be used for domestic purposes?
Answer:
Because in seawater, the strength of mineral salts is more.
![]()
Question 33.
Give the formula to express the strength of a solution. ‘ Mass of solute
Answer:
![]()
Question 34.
What is meant by 10% glucose solution?
Answer:
10%glucose solution means, 100 g solution contains 10 g of glucose.
Question 35.
What is the size of molecules of suspension?
Answer:
The size of molecules of suspension is more than 100 nm (10-7m).
Question 36.
By which method can the molecules of suspension be separated?
Answer:
The molecules of suspension can be separated by the filtration method.
Question 37.
Is the colloidal solution, homogeneous or heterogeneous?
Answer:
The colloidal solution is heterogeneous.
![]()
Question 38.
Give four examples of colloidal solutions used in our daily life.
Answer:
(i) Milk
(ii) Shaving cream
(iii) Toothpaste
(iv) Jelly.
Question 39.
What is the ‘Tyndall effect?
Answer:
When a narrow beam of light penetrates and enters the room through a small hole in the ceiling, the Tyndall effect can be noticed, because, inside the room, dust and smoke scatter light.
Question 40.
What is the size of colloid particles?
Answer:
The size of colloid particles is between 1 nm to 100 nm.
Question 41.
Give an example of the Tyndall effect
Answer:
When a fine beam of light enters in a room through a small hole in the roof, the Tyndall effect can be observed because the particles of dust and smoke scattered the light.
Question 42.
Can colloidal particles be seen by the naked eyes?
Answer:
No.
Question 43.
Give the name of a common colloid found in nature.
Answer:
Fog, is the common colloid found in nature, which is formed of water vapours present in the air.
Question 44.
What is the fundamental base of the classification of colloids?
Answer:
The fundamental base of classification of colloids is the state of dispersion medium and state of the dispersed phase.
Question 45.
Write two uses of colloids.
Answer:
In the manufacturing of medicines and in understanding the industrial processes.
![]()
Question 46.
Can water and mustard oil be separated by filtration?
Answer:
No, they can be separated either by evaporation or decantation method.
Question 47.
What is the principle of agitation?
Answer:
The method of separation of thin solid substance from a thick solid substance with a porous device is called as agitation method. No liquid is used in this method.
Question 48.
By which method contaminated water of rivers can be purified?
Answer:
By sedimentation contaminated water of rivers can be purified.
Question 49.
How is the melting point or boiling point of a substance useful?
Answer:
The purity or impurity of a substance can be known by these characteristic properties.
Question 50.
Which is the best method to separate solute substances in liquid?
Answer:
Distillation is the best method to separate solute substances in the liquid.
Question 51.
What is called as sublimation?
Answer:
The process in which a solid substance directly changes into the gaseous state is called sublimation.
Question 52.
Write the names of the two sublimated substances.
Answer:
Camphor and ammonium chloride are sublimated substances.
Question 53.
How can the mixture of water and kerosene oil be separated?
Answer:
The mixture of water and kerosene oil can be separated by a separating funnel.
Question 54.
How can cream (butter) can be skimmed from milk?
Answer:
Cream (butter) can be skimmed from milk by centrifugation method.
![]()
Question 55.
By which process distilled water is obtained?
Answer:
Distilled water can be obtained by the distillation process.
Question 56.
By which method can a mixture of ammonium chloride and sand be separated?
Answer:
A mixture of ammonium chloride and said can be separated by the sublimation method.
Question 57.
By which method water is purified in homes?
Answer:
By filtration method, water is purified in homes.
Question 58.
Which method helps to check adulteration in petrol?
Answer:
The evaporation method helps to check adulteration in petrol.
Question 59.
By which method, can a mixture of water and acetone be separated?
Answer:
By distillation method mixture of water and acetone can be separated.
Question 60.
Where is the distillation process applied?
Answer:
When there is much difference between the boiling points of the components of a mixture of two miscible liquids.
Question 61.
Which method is applied to separate different components of petroleum products?
Answer:
Different components of petroleum products can be separated by the fractional distillation process.
Question 62.
When is the fractional distillation process practised?
Answer:
When the difference of boiling points of two or more than two miscible liquids is less than 25 K.
Question 63.
By which method can different components of air be separated?
Answer:
By fractional distillation method, different components of air can be separated.
Question 64.
What is the boiling point of oxygen?
Answer:
The boiling point of oxygen is -183°C.
![]()
Question 65.
When the components of air are cooled down, which component changes into liquid at first?
Answer:
Oxygen.
Question 66.
By which method, pure copper sulphate is obtained from a sample of impure copper sulphate?
Answer:
By crystallisation method.
Question 67.
What is meant by physical changes?
Answer:
In physical changes, change takes place in the form of the substances and not in chemical form or in these changes a substance can be obtained again.
Question 68.
Give any two examples of physical changes.
Answer:
(i) Solution of sugar and water.
(ii) Solution of salt and water.
Question 69.
Can sugar be obtained from the solution of sugar?
Answer:
Yes. by evaporation of water from a solution of sugar, sugar can be obtained.
Question 70.
What is meant by chemical change?
Answer:
ln chemical change, one or more than one type of substance changes into one or many new substances or in these changes original substance cannot be obtained again.
Question 71.
Which are the two chemical changes taking place in daily life?
Answer:
(i) Formation of milk into curd.
(ii) Digestion of food.
Question 72.
What is an element?
Answer:
An element is a basic form of matter which cannot be broken into simple substances by chemical reactions.
![]()
Question 73.
Write any special property of an element.
Answer:
An element is composed of only a single type of atom.
Question 74.
How many elements are found in a gaseous state at room temperature?
Answer:
11 elements are found in a gaseous state at room temperature, like hydrogen and oxygen.
Question 75.
Write the names of a metal and a non-metal found in liquid state at room temperature.
Answer:
(i) Metal-Mercury.
(ii) Non-Metal-Bromine.
Question 76.
What is meant by alloys ?
Answer:
Elements displaying properties between metals and non-metals are called as alloys.
Question 77. Give names of any two alloys.
Answer:
(i) Boron
(ii) Silicon.
Question 78.
What is meant by compound?
Answer:
A substance formed by a chemical combination of two or more two elements in an equal ratio is called a compound.
Question 79.
Air is a homogeneous mixture of mainly which two gases?
Answer:
Air is mainly a mixture of oxygen and nitrogen gases because in air other gases are available in a little quantity.
Question 80.
What is called a heterogeneous mixture?
Answer:
A heterogeneous mixture is the one in which physically there are different parts and every part has different properties.
![]()
Question 81.
Which of the following are elements, compounds and mixtures:
(a) Na
(b) Soil
(c) Ag
(d) Sugar
(e) Urea
Answer:
(a) Element: (a) Na (c) Ag
(b) Compounds: (d) Sugar (e) Urea
(c) Mixture: (b) Soil
Question 82.
When two or more than two dements or compounds in any ratio combine together, then what is obtained ?
Answer:
Mixture is obtained when two or more than two elements or compounds in any ratio combine together.
Short-Answer Type Questions
Question 1.
What is called as a mixture? How many types of the mixture are there? Explain briefly.
Answer:
A mixture constitutes more than one substance (element/compound). A mixture can be separated by physical process into two or more two substances. Mixtures are mainly of two types:
1. Homogeneous Mixture: The mixture where composition remains constant everywhere, is called a homogeneous mixture e.g., a mixture of salt and water, a mixture of sugar and water.
2. Heterogeneous Mixture: The mixture in which physically there are different parts and each part is of different characteristics, is called a heterogeneous mixture e.g., a mixture of sodium chloride and iron peeling, a mixture of salt and sulphur and a mixture of water and oil.
Question 2.
Why is the solution of salt into water considered as a mixture but not a compound?
Answer:
Solution of salt into water is considered as a mixture but not a compound because:
(1) In salt water, salt and water can be separated by the distillation process.
(2) Saltwater displays the properties of of its both components – water and salt.
(3) Salt water has a different composition. By dissolving different quantity of salt into a definite quantity of water, salt water of different compositions can be obtained.
(4) Salt water does not have any fixed formula.
Question 3.
Give an example of such a mixture:
(i) Where both substances are compounds.
(ii) Where both element and compound are combined.
(iii) Where both are elements.
Answer:
(i) Such a mixture where both substances are compounds: On dissolving sugar in water, the syrup is formed which is a mixture in which sugar and water both are compounds.
(ii) Such a mixture where both element and compound are combined: Air is such a mixture in which oxygen and nitrogen are elements and carbon dioxide and water vapours both are compounds.
(iii) Such a mixture where both elements: Brass, copper and zinc are formed by mixing two elements.
Question 4.
What is called a solution ? Make a list of different types of solutions.
Answer:
A solution is a homogeneous mixture in which two or more two substances are there. Different types of solutions are as follows:
| State of solute | State of solvent | Example of solution |
| Solid | Solid | Gold and silver, copper and gold, combined metals (copper, bronze). |
| Solid | Liquid | Sugar-water, salt water. |
| Liquid | Solid | Mercury in silver. |
| Liquid | Liquid | Alcohol in water. |
| Gas | Gas | Air. |
| Gas | Liquid | Oxygen dissolved in water, carbon dioxide dissolved in water. |
Question 5.
Give any four characteristics of solutions
Answer:
Different characteristics of the solution are as follows:
(i) Solution is a homogeneous mixture.
(ii) As molecules of solution are smaller in diameter than lnm (10-9 m), so they cannot be seen with naked eyes.
(iii) Due to their smaller size, solutions do not scatter the passing over the beam of light, thus no path of light is seen in solutions.
(iv) Particles of solute cannot be separated from the solution by the filtration method. On leaving the solution, untouched even then the particles of solute do not settle down, thus the solution is stable.
![]()
Question 6.
What are aqueous and non-aqueous solutions?
Answer:
Aqueous Solutions: These are those solutions which are prepared by dissolving substances into the water; like syrup and soda water etc.
Non-aqueous Solutions: These are those solutions which are prepared by dissolving apart from water in other solvents (like alcohol, acetone etc.) e.g., solution in benzene of tincture iodine nap/itfiafene.
Question 7.
Why is it not possible to identify solute and solvent particles separately in a solution?
Answer:
Particles of solute are so closely combined with a solvent that they cannot be separately identified. Solutions are homogeneous, thus the composition of solutions is uniform. If salt-solution and sugar-solution are mixed, as the result in the obtained homogeneous solutions both solutions combine with each other well and to make difference between basic solute and solvent is not possible.
Question 8.
How is the strength of a solution expressed? Explain with an example with its meaning.
Answer:
The strength of solutions is expressed in the context of the quantity of solute present in the mass or volume of a given solution or in the context of the quantity of dissolved solute in the mass or volume of a given solvent The strength of solutions can also be expressed in the form of per cent volume of solutes which give the mass of solutes in the 100 mass per unit. Its mass unit is gram.
![]()
For example, if we take a solution of 10% glucose (C6H12O6) by mass. It contains 10 g of glucose in 100 g of solution. It can also be called in the form as 10 g of glucose in 90 g of water. If something is not said in a special manner, then the meaning of per cent in the form of mass is per cent and water is solvent in it.
Question 9.
Define saturated solution, unsaturated solution and supersaturated solution.
Answer:
Saturated Solution: When at a given temperature in a solution the extra solute that gets dissolved in it beyond its capacity, then it is called a saturated solution. In this solution, dissolved and undissolved solutes are in equilibrium together.
Unsaturated Solution: If in a solution, the quantity of a solute already present in it is less than the saturated level, then the solution is called an unsaturated solution.
Supersaturated Solution: If in a solution, the strength of the solution is more than the saturated strength, this solution is called a supersaturated solution.
Question 10.
A certain solution, in 320 g of water keeps 40 g of salt. Give the strength of solution.
Solution:
Mass of solute substance (salt) = 40 g
Mass of solvent (water) = 320 g
Mass of solution = Mass of solute substance + Mass of solution = 40g + 320 g = 360 g
![]()
\(\frac {40}{360}\) × 100 = 11.1%
Question 11.
Define suspension and write its different characteristics.
Answer:
Suspension: Suspension is a heterogeneous mixture, in which the particles of a solute substance do not dissolve, rather they remain suspended in the equilibrium of the medium.
Its main characteristics are as follows:
(i) It is a heterogeneous mixture.
(ii) The suspended particles are bigger than 100 nm (10-7m) as a result they can be seen with naked eyes.
(iii) These suspended particles scatter the beam of light, due to this its path is known.
(iv) Suspension is temporary. By filtration method, its particles can be separated from the mixture.
![]()
Question 12.
What is the difference between solution and colloid?
Answer:
Following is the difference between solution and colloid:
Solution:
1. Solution does not reflect light.
2. Solution is a homogeneous mixture.
3. They are totally transparent.
4. In the real state the size of particles of the solution is less than 10-9m.
5. They do not display the Tyndall effect.
Colloid:
1. Colloid reflects light.
2. Colloid is a heterogeneous mixture.
3. Colloid is a little transparent.
4. The size of colloid particles is between 10-9 to 10-7 m.
5. They display the Tyndall effect.
Question 13.
What is the difference between solution and suspension?
Answer:
Following is the difference between solution and suspension:
Solution:
1. It is homogeneous.
2. It is transparent.
3. Its particles are comparatively smaller in size.
4. It passes through filter paper and leaves no residue behind.
5. Its particles are invisible.
Suspension:
1. It is heterogeneous.
2. It is opaque (hazy).
3. Its particles are comparatively bigger in size.
4. Its particles cannot pass through filter paper.
5. Its particles can be seen with eyes or a compound microscope.
Question 14.
What happens when a beam of light is passed through a colloidal solution?
Answer:
When a beam of light is passed through a colloidal solution, its path gets illumined, because the size of the colloidal particles is bigger, therefore these particles scatter the falling light on them in all directions. This is called as Tyndall effect. Colloidal solution and the actual solution are differentiated on this basis. For example, milk shows the Tyndall effect. In daily life when a beam of light passes through a small hole into a room, then we can notice Tyndall’s effect there.
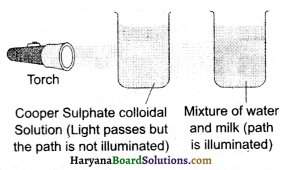
Question 15.
What is meant by the Brownian movement?
Answer:
Colloidal molecules always keep travelling in zig-zag paths in all directions. The unending movement of colloidal molecules in zig-zag paths is called as Brownian movement.
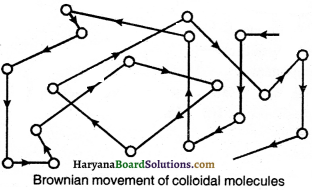
Question 16.
Describe the characteristics of colloids.
Answer:
The characteristics of colloid are as follows:
(i) It is a heterogeneous mixture.
(ii) The size of colloidal molecules is between lnm to 100 nm and they cannot be seen with eyes.
(iii) They are so large that they scatter the ray of light and make Its path visible.
(iv) When they are left standstill, then they do not settle at the bottom which means they are stable.
(v) They cannot be separated by a filtration process but can be separated by the centrifugation method.
![]()
Question 17.
How are colloids classified? Explain by giving examples.
Answer:
Colloids are classified according to the state (solid, liquid or gas) of the dispersing medium and the dispersed phase, which is clarified by following examples:
| Dispersing Medium | Dispersed Phase | Type | Example |
| Gas | Liquid | Aerosol | Mist, fog, cloud |
| Gas | Solid | Aerosol | Smoke, automobile exhaust |
| Liquid | Gas | Foam | Shaving cream |
| Liquid | Liquid | Emulsion | Milk, face cream |
| Liquid | Solid | Solid | Mud, milk of magnesia |
| Solid | Gas | Foam | Foam rubber, sponge, pumice |
| Solid | Liquid | Gel | Jelly, cheese, butter |
| Solid | Solid | Solid sol | Coloured gemstone, milky glass |
Question 18.
Give any five differences between compound and mixture.
Answer:
Five differences between compound and mixture are as follows:
Compound:
1. It is composed of two or more than two elements in a definite ratio by chemical combination.
2. It is homogeneous.
3. Its physical and chemical properties are definite.
4. The components of compound cannot be seen separately.
5. Its components cannot be separated by physical methods.
Mixture:
1. It is composed of two or more two substances in any ratio combined together. It is not a chemical combination.
2. It is heterogeneous.
3. Its properties are not definite.
4. Its components can be seen separately.
5. Its components can be separated by physical methods.
Question 19.
What purposes are the components of mixtures separated for?
Answer:
Components of mixtures are separated for the following purposes:
(i) To separate unwanted components.
(ii) To separate any harmful component.
(iii) To obtain pure sample of any substance.
(iv) To obtain any useful component.
Question 20.
Classify the following into metals, non-metals and metalloids:
Silicon, Germanium, Iodine, Sodium, Iron, Carbon.
Answer:
Metals: Sodium, Iron
Non-Metals: Iodine, Carbon
Metalloids: Silicon, Germanium
![]()
Question 21.
Explain the threshing method.
Answer:
A peasant, when stands at a raised platform and the method that he adopts to separate chaff and wheat grains, it is called as threshing. Chaff being lighter in weight flies away with wind and the wheat grains being heavier in weight, directly fall onto the ground.

Question 22.
Explain briefly ‘weaning with hands’ a method of separation.
Answer:
Separating of unwanted materials like-fine pieces of stone pebbles from wheat, rice and pulses, by weaning with hands is called as ‘weaning or gleaning’. This method is also adopted to wean fruit and vegetables.

Question 23.
What is the evaporation process? Explain.
Answer:
If in a liquid, some other solute substance is got dissolved and if we are supposed to separate the components of that mixture, then the mixture is evaporated by heating in the sun. The liquid evaporates in the form of water vapours and the miscible solute is left behind. This method is called as evaporation. Salt is obtained from seawater by means of this method.
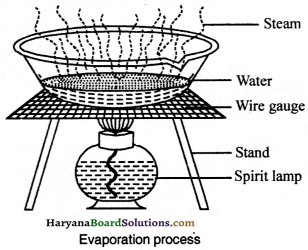
Question 24.
What is called a centrifugation method?
Answer:
In this method, that mixture is rotated all around in an enclosed vessel which has very small suspended particles. The heavy material settles down at the bottom of the vessel and the lighter material comes up. This is called as centrifugation method. In dairy, the cream is skimmed from milk by this method.
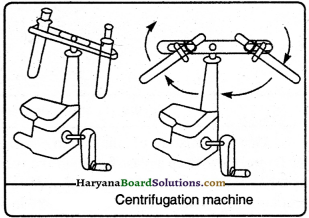
Question 25.
Describe the uses of the centrifugation method in day-to-day life.
Answer:
Uses of the centrifugation method in day-to-day life are as follows:
(i) In the diagnostic laboratory, this method is adopted to test blood and urine.
(ii) It is adopted in dairy and houses to extract butter from cream.
(iii) It is adopted to squeeze water from wet clothes in washing machines.
![]()
Question 26.
How will you separate kerosene oil from water ?
Answer:
Water and kerosene oil, both are immiscible liquids. Both liquids form different layers. Kerosene oil being lighter, floats on the surface of water. Both the components can be separated by separating funnel according to the given figure. Invert the mixture into the separating funnel. After sometime both the liquids will form separate layer. By opening the stopcock of the funnel the water is separated into the beaker kept just below to the stem of the funnel, whereas kerosene oil leaves behind in the funnel.
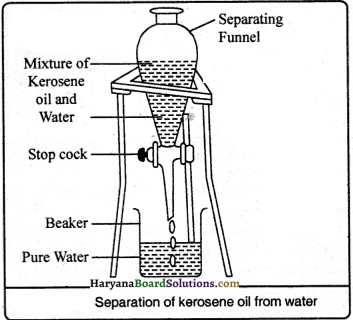
Question 27.
Which principle is used in a separating funnel to separate mixture of two immiscible liquids ? Give any two uses of it.
Answer:
The principle that is used in the separating funnel, it is that two immiscible liquids according to their densities get separated into two separate layers and by opening the stopcock are got separated.
Following are the two uses of it:
(i) In separation of two components i.e., water and oil.
(ii) During refining of a metal in separation of iron. By this method the lighter slug is collected from the top and molten iron leaves behind at the lower surface of the furnace.
Question 28.
What is sublimation ? How will you separate the mixture of salt and ammonium chloride ?
Answer:
The process of changing a solid material directly into gaseous state by heating, is called as sublimation. Generally, on heating, solid materials melt away, but, certain solid materials do not change into liquid on heating. They directly convert into gaseous state, such materials are called as volatile materials. Ammonium chloride, camphor, iodine and nephthalene are volatile materials.
Separation of Ammonium Chloride and Salt Mixture: To separate the mixture of ammonium chloride and salt, sublimation method is used.
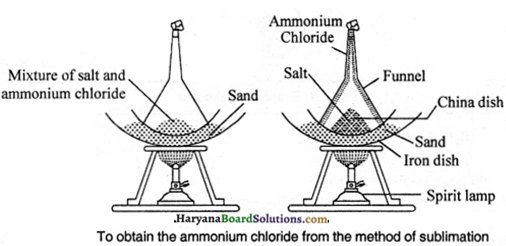
Place the mixture of ammonium chloride and salt in China dish and put it on a tripod stand according the figure. Now, keep a glass funnel in an inverted position on the China dish as shown in the figure. Close the mouth of the stem of the funnel with a cotton plug. Start heating the China dish with a spirit lamp at it. On heating ammonium chloride will convert in vapour state. The vapours deposited on the inner wall of the funnel will again come back into solid state and salt will remain behind in the China dish. By this method the mixture of ammonium chloride and salt can be separated.
![]()
Question 29.
What do you understand bv chromatography ? What are its uses in daily life ?
Answer:
Chromatography is that process which is used to separate those solute materials which are dissolved in just one kind of solvent. It is used to separate the following:
(i) To separate the colours in dyes.
(ii) In separation of colour from natural colours.
(iii) To separate sugar from urine.
(iv) To separate medicine from blood.
Question 30.
What is called as distillation ? Where is it used ?
Answer:
Conversion of a liquid into vapour by heating and again converting it into liquid by cooling is called as distillation. This distilled water used by the doctors obtained by this method. This method is used to separate mixture of those two soluble liquids which have much difference between their boiling points (More than 25 k).
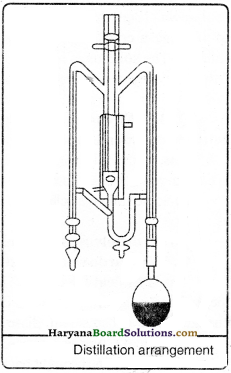
Question 31.
Where is fractional distillation practised ? How is it different from distillation process ?
Answer:
Fractional distillation is practised when the boiling point difference between two or more than two miscible liquids is less than 25 K which are mixed together. For example, separation of different gases from the air and separation of different components from petroleum products. Its apparatus resembles to that of the common distillation process-apparatus. Only a fractionating column is installed in between distillation flask and condenser. The common fractionating column is equipped with a tube which is filled up with glass powdered pieces. These glass powdered pieces provide surface to vapours to cool down and condense as shown in the figure.
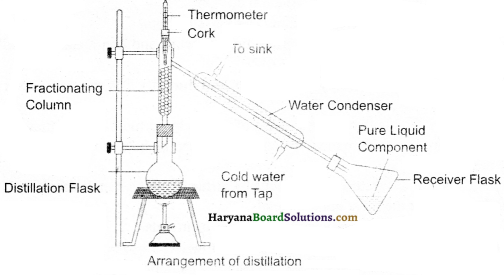
Question 32.
What is called as crystallisation ? Give its two usages.
Answer:
Obtaining of pure and of definite sized pieces of a solid material from a mixture of solution is called as crystallisation. Alum, common salt, copper sulphate (blue vitrol) can be obtained in pure state through this method.To obtain crystals, firstly an impure sample is dissolved in maximum quantity in the hot liquid, then this solution is filtered and the remaining impurities are removed. This solution is let cooled down for sometime and therefore, pure solid crystals are obtained.
Minor Usages:
(i) In purifying the salt obtained from sea water.
(ii) In separation of alum from impure sample.
Question 33.
How will you prepare a big crystal from the pulverised sugar ?
Answer:
To obtain a big crystal from the pulverised sugar, prepare a solution of it in hot water. This solution is kept to cool down. Then, the solution is filtered and with the help of a thread a crystal of sugar is got suspended into this solution. The solution is left standstill in the open as usual. After a few days we notice the sugar crystal starts increasing in its size. Thus, we obtain a big crystal of sugar.
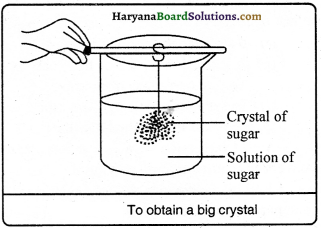
Question 34.
Why is crystallisation method considered better to common evaporation method ?
Answer:
In the following respect, crystallisation method is considered better to common evaporation method:
(i) Some solid particles breakdown or some get spoiled as sugar.
(ii) On dissolving solute material into solvent there are left behind some impurities in solution.
Question 35.
What do you understand by physical and chemical changes ? Make it clear by giving examples.
Answer:
Physical Change: These are temporary in which change takes place only in physical state. In these changes chemical changes do not occur and they can be converted into their basic state. For example, changing of water in steam, dissolving of sugar in water, dissolving of salt into water etc.
Chemical Change: These are permanent in which along with physical change chemical changes too occur. They cannot be brought back into their basic state. For example, corroding of iron, igniting of magnesium wire in presence of oxygen.
![]()
Question 36.
Define and classify an element into different groups.
Answer:
An element is that basic state of matter, which cannot be further subdivided into tiny particles through chemical process. This can be divided into the following three groups:
1. Metals: Gold, Silver, Copper, Iron, Sodium etc.
2. Non-Metals: Hydrogen, Oxygen, Iodine, Carbon, Coal etc.
3. Alloys: Elements lie in between the properties of metals and non-metals are called as alloys like- boron, silicon.
Question 37.
What do you understand by malleability and ductility ? Write names of two malleable and ductile metals each.
Answer:
Malleability: This is that characteristic of metals by virtue of which they are battered into thin sheets. Gold and silver are the most malleable metals. They can be battered with hammer yet into very thin foils than a leaf-paper.
Ductility: This is that characteristic of metals by virtue of which metals are drawn in the form of long cables. Silver and copper are the most ductile metals.
Question 38.
Write down the salient physical properties of metals.
Answer:
Following are the salient physical properties of metals:
1. Physical State: Except mercury, all other metals are solid at normal temperature.
2. Metallic lustre: All metals have a peculiar metallic lustre or shining.
3. Structure: In the outermost shell of metals there are 1, 2 or 3 electrons.
4. Conductivity: Metals are generally good conductors of heat and electricity.
5. Malleability and Ductility: Metals are generally malleable and ductile.
6. Solidity: Generally metals are solid. Sodium and potassium are soft metals, they can be cut with knife.
7. Density: Except, sodium and potassium, the density of all the metals generally high.
8. Melting point and Boiling point: The melting points and boiling points of metals are comparatively high.
Question 39.
Give a brief account of information regarding elements available so far.
Answer:
Information regarding elements available so far is as follows :
(i) The number of elements known so far is more than 112. Out of them 92 elements are natural, whereas rest of them are man-made.
(ii) Maximum elements are solids.
(iii) 11 elements are gases at room temperature.
(iv) Two elements, bromine and mercury are liquid at room temperature.
(v) Gallium and cesium can remain in liquid state at the temperature above 303K.
![]()
Question 40.
Explain the Conduction method.
Answer:
When the size of the components of mixture is different, then the sieves with different pores are used to separate components. This method is known as conduction method. In separation of bran from flour, separation of cashewnuts in cashewnut industries, separation of different pearls of different sizes by the goldsmiths and separation of different foodgrains by the peasants, in all these cases conduction method is applicable.
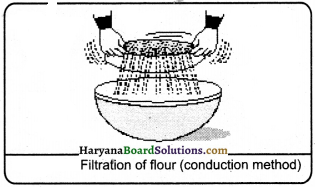
Question 41.
What is magnetic separation method ?
Answer:
By making use of magnetic characteristic into real practice to separate the iron fillings or iron particles from a mixture is called as magnetic separation method.
Mineral ore like: iron ore is obtained from underneath the ground by this method.
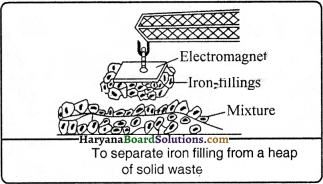
Question 42.
State briefly the method of sedimentation.
Answer:
At times, in the water from ponds or lakes the suspended particles like sand or soil particles present in the dirty water do not quickly settle down at the bottom that is, it takes times to decant them. Therefore, in order to make such particles settle down at the bottom at the earliest or to make the particles heavier in weight, alum is mixed into the water and water is decanted soon. It is called as sedimentation method. The water of pond or lake can be purified by this method.
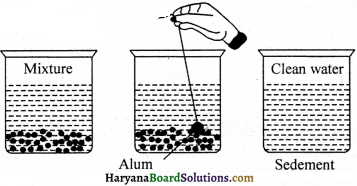
Essay Type Questions
Question 1.
Explain the method to obtain different gases from air.
Answer:
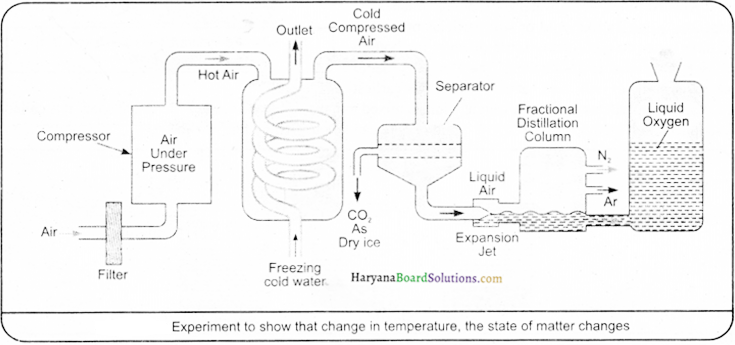
Air is a homogeneous mixture of different gases and its components can be separated by fractional distillation. Different stages or phases of this method have been shown diagrammatically ahead:
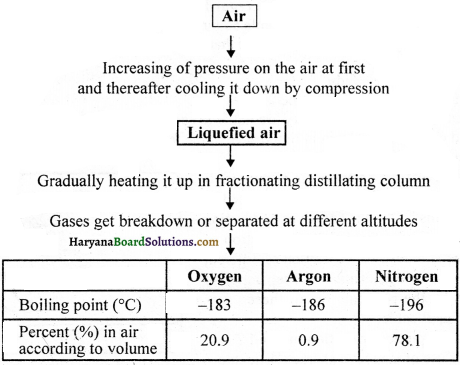
For instance, if we want to obtain oxygen gas from the air (according to the figure), then we will have to isolate other gases present in the air. In order to obtain liquefied air, initially pressure is increased on the air and then by decreasing temperature and cooling it down and it is compressed. This liquefied gas is further heated up in fractionating distillating column, where all gases get separated in accordance with their boiling points at different altitudes, as it is shown in the fig. below:
Question 2.
Name the method applicable to separate the following mixtures respectively:
(i) Wheat grains, sugar crystals and chaff.
(ii) Rice, grams and iron powder.
(iii) Sand, phaseolies mungo (dal mash) and chaff.
(iv) Sand, camphor and iron powder.
(v) Sand, sugar and iron powder.
Answer:
(i) This mixture can be separated by more than one methods. Mixture of chaff is separated by threshing method. After that the mixture is filtered by immersing it into water. Sugar due to possessing solution quality makes solution in water and the wheat grains leaves behind on the sieve. They are got dried up. Thereafter, solution is heated up in the porcelain dish. On heating water evaporates in the form of steam and sugar leaves behind in the porcelain dish. Hence, all the three components get separated.
(ii) To separate this mixture too more than one methods are applied. Firstly, creep the magnet into the mixture, with the result the iron powder get stuck to the magnet and is got separated. Again, the remaining rice and grams can be separated by filtration method. In this method, rice will leave at the bottom and grams will come up.
![]()
(iii) To separate this mixture, at first chaff is separated by threshing method. Sand and phaseolies mungo will be left behind. These can be separated by filtration process.
(iv) Firstly, magnet is moved above the mixture, the iron powder gets stuck to the magnet and thus is got separated. After that, the mixture is separated by sublimation process. Camphor turns into vapours, which get deposited on the inner walls of the funnel when they cool down and sand remains behind in the vessel. Thus, all the three components get separated.
(v) The iron powder is got separated from the mixture by using the magnet. Then mixture is dissolved into water. Sugar gets dissolved in water. Thereafter it is filtered, sugar being solute goes down to the bottom in the form of solution, and the sand leaves behind on the filter-paper. Then this solution is heated up. On heating water evaporates and sugar is left behind in the container.
Question 3.
Explain water supply system in towns diagrammatically, also tell which separating methods are adopted ?
Answer:
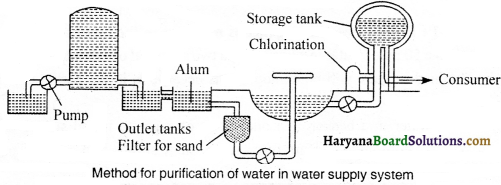
In towns, water is supplied by water-works. A flow diagram of typical water works is shown in the figure. In these water works, by means of distillation decantation, sedimentation and filtration processes unwanted material is separated from water. In loading process, you will certainly be reminded of usage of alum. In water works, to kill the harmful germs, chlorine is used. This very’ purified water is supplied in houses through pipe-lines.
Question 4.
What is the difference between ‘distillation’ and ‘fractional distillation’ ?
Answer:
Distillation:
1. Mixture is heated upto the temperature of the component with least boiling pointfor sometime.
2. Only single component evaporates from the mixture at a time.
3. Mixture has to be heated up again and again at different temperatures.
4. The vapours formed by heating the mixture are condensed by passing them through the delivery tube.
5. Pure liquid is not obtained just at the first time.
6. On heating the mixture at different temperatures components are obtained.
Fractional distillation:
1. Mixture is heated upto the temperature of the liquid with maximum boiling point.
2. All the evaporating components of the mixture evaporate at a time.
3. Mixture is only once evaporated by heat process.
4. While heating the mixture, the steam that is formed is sent into the fractionating column.
5. Pure components are obtained just atthe first time.
6. On sending the steam of the mixture into fractionating columns, at different fractionating levels, different fractions are obtained.
![]()
Question 5.
Differentiate between the physical properties of metals and non-metals.
Answer:
Following are the differences between the physical properties of metals and non-metals:
| Physical properties | Metals | Non-Metals |
| 1. Structure | In the outermost shell (orbit) 1, 2 or 3 electrons exist. | They have 4 to 8 electrons in the outermost shell. |
| 2. Physical state | These are generally found in solid state (except mercury). | They are found in three states (solid, liquid and gas). |
| 3. Lustre | Metals have a peculiar metallic lustre. | They have no lustre. |
| 4. Conductivity | Metals are generally good conductors of heat and electricity. | They are generally bad conductors. Graphite is an exception. |
| 5. Malleability and ductility | Metals are generally | Non-metals are generally brittle. |
| 6. Flardness | malleable and ductile. | These are soft. |
Practical Work:
Experiment – 1:
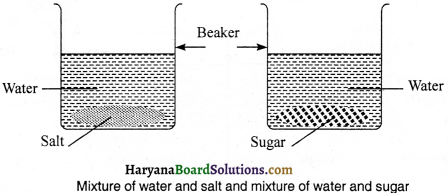
Prepare two homogeneous mixtures in the laboratory.
Procedure:
(i) Take 50 ml of water in a beaker, add two spoonful of salt into it and stir the solution with a spoon. Thus, the obtained mixture will be homogeneous because in it, the particles of solute and solvent cannot be identified separately.
(ii) Take 50 ml of water in a beaker, add one spoon of sugar to it and stir it. Thus, by stirring the obtained solution will be homogeneous mixture, since, in it solute and solvent particles cannot be separately identified
Experiment – 2:
Prove with an experiment that solution is homogeneous.
Procedure:
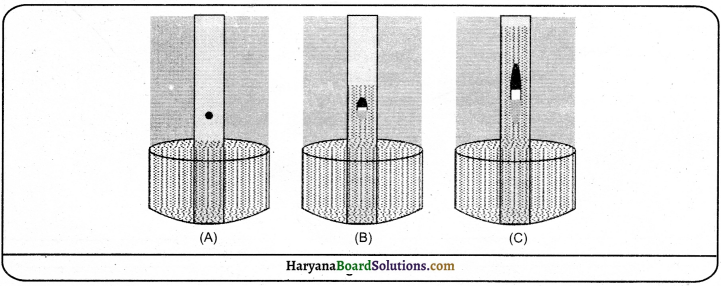
Take a solution of salt and water. Filter it through filter paper and taste the filtered solution. It will be salty as before. Now, look at the filter paper. It does not have any residue. Thus, it proves that solutions are homogeneous which escape through the filter paper and leaves no residue.
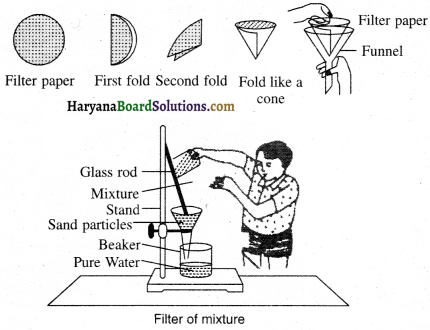
Experiment- 3:
How can components of colour (dye) be separated from blue or black ink ?
Procedure:
Take a beaker half-filled with water, now keep a watch glass on its mouth as shown in the figure. Trickle down a few drops of ink on it. Now, start heating up the beaker. We don’t want to directly heat the ink. You will notice evaporation taking place in the watch glass. Heating is done constantly till evaporation. When we do not notice any further change in the watch glass, then we stop heating. Thus, a blue or black component remains left behind in the watch glass as a residue. In this process in the ink-water coloured mixture water gets evaporated by means of evaporation.
Experiment – 4:
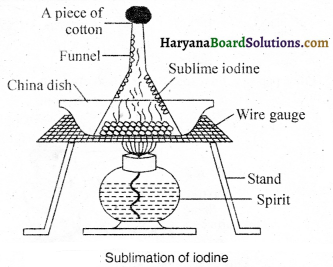
How will you separate iodine and sand from its mixture ?
Procedure:
The mixture of iodine and sand is separated by sublimation process. In this process, the mixture is put into a porcelain dish and a separating funnel is kept on it in an inverted position. The opening of the stem of the funnel is blocked with a cotton plug. Now, this mixture is heated from at the bottom. On heating iodine directly converts into vapour state which on cooling down, gets deposited on to the inner walls of the funnel and the sand is left behind in the dish. Thereby, iodine is scratched down from the funnel. Thus, the components of mixture get separated.
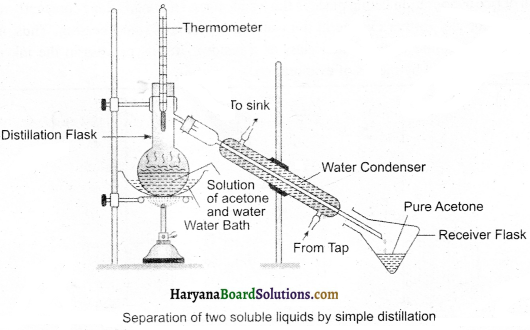
Experiment – 5:
How is acetone and water separated from their solution in the laboratory ?
Procedure:
(i) Pour the mixture into the distillation flask. Joins a thermometer with this.
(ii) Set the apparatus according to the given diagram.
(iii) Gradually heat up the flask and carefully keep eyes on the thermometer.
(iv) Acetone evaporates and on condensing, can be collected into the vessel by condensation after taking
(v)Water is remain left in the distillation tiask. Thus, acetone and water will get separated.
Quick Review of the Chapter
1. Which of the following is a pure material?
(A) sugar
(B) milk
(C) air
(D) pond water
Answer:
(A) sugar
2. The size of the particles in the solution is:
(A) smaller than 10-9m
(B) smaller than 10-7m
(C) smaller than 10-5m
(D) smaller than 10-2m
Answer:
(A) smaller than 10-9m
3. The size of the particles in colloid is:
(A) 10-5m to 10-4m
(B) 10-6 to 10-5m
(C) lCr9mto 10-7m
(D) more than 10-7m
Answer:
(C) 10-9m to 10-7m
![]()
4. The size of particles in suspension is:
(A) more than 10-5m
(B) more than 10-7m
(C) more than 10-4m
(D) more than 10-3
Answer:
(B) more than 10-7m
5. An example of suspension is:
(A) salt solution
(B) ink
(C) paint
(D) milk
Answer:
(C) paint
6. Butter is extracted from curd by:
(A) centrifugation method
(B) fractional distillation process
(C) evaporation method
(D) crystallisation method
Answer:
(A) centrifugation method
7. Salt is separated from sea-water by:
(A) sifting/agitation method
(B) evaporation method
(C) centrifugation method
(D) sublimation process
Answer:
(B) evaporation method
8. In a mixture of salt and camphor, camphor and salt are separated by :
(A) evaporation method
(B) centrifugation method
(C) sublimation process
(D) filtration method
Answer:
(C) sublimation process
![]()
9. From the mixture of copper sulphate and iron powder, its components can be separated
(A) separating funnel
(B) sublimation process
(C) magnetic process
(D) centrifugation method
Answer:
(C) magnetic process
10. In a mixture of water and oil, water and oil are separated by :
(A) separating funnel
(B) sublimation
(C) evaporation
(D) filtration
Answer:
(A) separating funnel
11. Which of these is not a compound ?
(A) blood
(B) CO2
(C) methane
(D) soap
Answer:
(A) blood
12. Which of these is not a mixture ?
(A) rock salt
(B) blood
(C) coal
(D) soap
Answer:
(D) soap
![]()
13. The dirty pond water is purified by:
(A) decantation
(B) sedimentation
(C) centrifugation
(D) filtration
Answer:
(B) sedimentation
14. In water, solute is:
(A) sand
(B) sulphur
(C) salt
(D) soot
Answer:
(C) salt
15. Solvent present in tincture iodine is:
(A) water
(B) rose elixir (gulabjal)
(C) carbon dioxide
(D) alcohol
Answer:
(D) alcohol
16. Number of known elements is:
(A) 92
(B) 108
(C) 112
(D) 118
Answer:
(C) 112
17. At room temperature number of elements found in gaseous state is:
(A) 06
(B) 09
(C) 11
(D) 23
Answer:
(C) 11
![]()
18. Physical change is:
(A) evaporation of water
(B) baking of wheat cake
(C) ripening of fruit
(D) rusting of iron
Answer:
(A) evaporation of water
19. Which of these is a metalloid ?
(A) sodium
(B) silicon
(C) iodine
(D) carbon
Answer:
(B) silicon
20. By which method can the mixture of iodine and sand be separated ?
(A) centrifugation
(B) distillation
(C) sublimation
(D) filtration
Answer:
(C) sublimation
21. In the outermost shell of metals the number of electrons not present is:
(A) 1
(B) 2
(C) 3
(D) 4
Answer:
(D) 4
![]()
22. Non-metal which is a good conductor of electricity is :
(A) iodine
(B) sulphur
(C) graphite
(D) phosphorus
Answer:
(C) graphite
23. A matter composed of particles of same kind is called :
(A) pure matter
(B) mixture
(C) solution
(D) impure matter
Answer:
(A) pure matter
24. A mixture whose composition is same, is called :
(A) homogeneous mixture
(B) heterogeneous mixture
(C) colloidal
(D) suspension
Answer:
(A) homogeneous mixture
25. Which of the following is not a homogeneous mixture ?
(A) solution of sugar in water
(B) mixture of sand and salt
(C) solution of salt in water
(D) mixture of water and alcohol
Answer:
(B) mixture of sand and salt
![]()
26. The number of naturally occurring elements is:
(A) 112
(B) 22
(C) 92
(D) 108
Answer:
(C) 92
27. Which of the following is not an element ?
(A) sodium
(B) silver
(C) tin
(D) soap
Answer:
(D) soap
28. Which of the following is not a compound ?
(A) calcium carbonate
(C) carbon dioxide
Answer:
(D) air
29. Which one is a mixture ?
(A) methane
(C) blood
Answer:
(C) blood
30. The solution obtained after dissolving a solute in any other liquid besides water is called
(A) non-aqueous solution
(B) homogeneous solution
(C) heterogeneous solution
(D) aqueous solution
Answer:
(A) non-aqueous solution
![]()
31. Main constituents of air are:
(A) C02 and Ar
(B) N, and H2
(C) C02 and vapour
(D) He and Ne
Answer:
(B) N2 and H2
32. If the quantity of a solute gets reduced less than saturation in a solution, then it is called
(A) saturated solution
(B) supersaturated solution
(C) unsaturated solution
(D) homogeneous solution
Answer:
(C) unsaturated solution
33. Which the following is property of Metals?
(A) Ductivity
(C) metallic lusture
(B) malleability
(D) all of the above
Answer:
(D) all of the above
34. Which of the following element is non-metal?
(A) Bromine
(C) Silver
(B) Gold
(D) Sodium
Answer:
(A) Bromine
35. Which of the following metal exist in liquid state at room temperature?
(A) Gold
(B) Silver
(C) Mercury
(D) Bromine
Answer:
(C) Mercury