Haryana State Board HBSE 10th Class Science Solutions Chapter 4 Carbon and Its Compounds Textbook Exercise Questions and Answers.
Haryana Board 10th Class Science Solutions Chapter 4 Carbon and Its Compounds
HBSE 10th Class Science Carbon and Its Compounds Textbook Questions and Answers
Question 1.
Ethane, with the molecular formula C2H6 has ……………..
(a) 6 covalent bonds
(b) 7 covalent bonds
(c) 8 covalent bonds
(d) 9 covalent bonds.
Answer:
(b) 7 covalent bonds
![]()
Question 2.
Butanone is a tour-carbon compound with the functional group
(a) carboxylic acid
(b) aldehyde
(c) ketone
(d) alcohol
Answer:
(c) ketone
Question 3.
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
Answer:
(b) the fuel is not burning completely.
Question 4.
Explain the nature of the covalent bond using the bond formation in CH3Cl.
Answer:
The atomic numbers of carbon, hydrogen and chlorine are 6, 1 and 17 respectively. Their electronic configurations are: Carbon C (2, 4), Hydrogen H (1), Chlorine Cl (2, 8, 7)
1. As can be seen, carbon requires 4 electrons to complete its octet, hydrogen requires 1 electron to complete its duplet and chlorine requires 1 electron to complete its octet.
2. Therefore, carbon shares its four electrons, one electron each with three hydrogen atoms and one electron with a chlorine atom to form four covalent bonds and complete its octet.
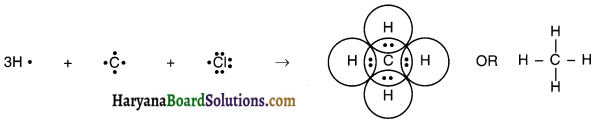
3. Thus, carbon attains the stable electronic configuration of nearest noble gas neon, hydrogen attains the electronic configuration of noble gas helium, while chlorine attains the electronic configuration of noble gas argon.
4. Chloromethane forms three C – H covalent bonds and one C – Cl covalent bond.
![]()
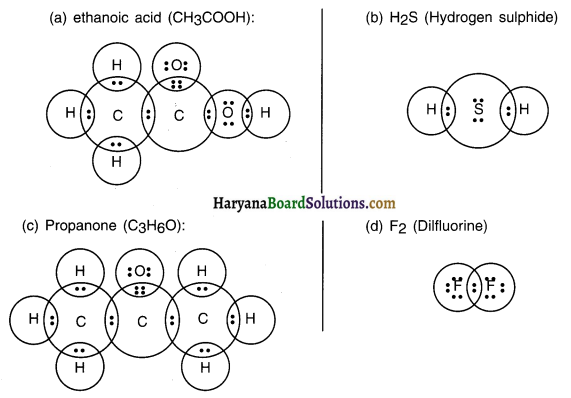
Question 5.
Draw the electron dot structures for
(a) ethanoic acid
(b) H2S
(c) propanone
(d) F2
Answer:
Question 6.
What is a homologous series? Explain with an example.
Answer:
Homologous series:
- The series of organic compounds in which a particular functional group attaches to the carbon chain in place of hydrogen atom is called a homologous series.
- Each compound of the homologous series differs from its previous or later compound by (CH2).
- For example, the alkanes namely methane (CH4), ethane (C2H6), propane (C3H8) and so on form a homologous series with a definite difference of CH2.
- Similarly, CH3OH, C2H5OH, C3H7OH is the homologous series of alcohols with functional group
- OH and with a definite difference of CH2 between each compound.
![]()
Question 7.
How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties? Difference between ethanol and ethanoic acid:
| Ethanol |
Ethanoic acid |
| Physical properties 1. It has a bitter smell 2. Its melting point is 156 K whereas boiling point is 351 K. |
Physical properties 1. It has pungent smell 2. Its melting point is 290 K whereas boiling point is 391k. |
| Chemical properties 1. On adding it to sodium bicarbonate, CO2 gas is produced 2. The colour does not disappear on adding alkaline potassium permanganate. |
Chemical properties 1. CO2 gas is not produced 2. On adding alkaline potassium permanganate the colour disappears. |
Question 8.
Why does micelle formation take place when soap ¡s added to water? Will a micelle be formed in other solvents such as ethanol also?
(a) Micelle formation:
(b) Solubility in ethanol:
Answer:
1. Micelle formation does not take place in all types of solvents. Micelles are formed only in those solvents in which soap is soluble.
2. Since soap is not soluble in ethanol, micelles will not be formed.
Question 9.
Why are carbon and its compounds used as fuels for most applications?
Answer:
Carbon and its compounds have high thermal capacity. On burning them, a large amount of heat is used which can be put to several use, Hence, carbon and its compounds are used as fuels for most applications.
![]()
Question 10.
Explain the formation of scum when hard water is treated with soap.
Answer:
1. Hard water contains salts of calcium and magnesium.
2. When soap molecules come in contact with these salts, they form a curdy white precipitate, insoluble in water. This is called scum.
Question 11.
What change will you observe you test soap with litmus paper (red and blue)?
Answer:
Soap is alkaline (basic) in nature. Hence, it does not show any effect on the blue litmus paper. However, soap will turn red litmus paper blue.
Question 12.
What is hydrogenation? What is its Industrial application?
Answer:
1. A reaction in which adding one molecule to an organic compound gives a new but single organic compound is called an addition reaction.
2. For example, on adding hydrogen to an unsaturated (alkene or alkyne) hydrocarbon in the presence of catalysts such as palladium or nickel gives a single but saturated (alkane) product. This reaction is called an addition reaction.
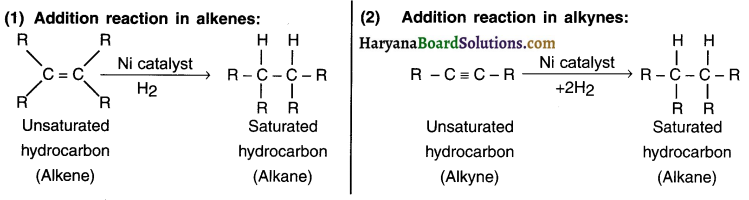
Question 13.
Which of the following hydrocarbons undergo addition reactions?
C2H6, C3H8, C3H6, C2H2 and CH4.
Answer:
1. Addition reaction occurs in unsaturated hydrocarbons.
2. Out of the given compounds only C3H6 and C2H2 are unsaturated hydrocarbons. Hence, only these compounds Will undergo additional reaction.
![]()
Question 14.
Give a test that can be used to differentiate between saturated and unsaturated hydrocarbons.
Answer:
1. Alkaline potassium permanganate (KMnO4) can be used to test if the compound is saturated or unsaturated.
2. Put some butter in one test tube and cooking oil in another. Add KMnO4 to both.
3. The pink colour of KMnO4 will disappear in oil but not in butter. Hence, we can conclude that cooking oil is unsaturated whereas butter is not.
Question 15.
Explain the mechanism of the cleaning action of soaps.
Answer:
Detergents:
- Detergent is a chemícal substance used for cleaning purposes.
- A molecule of detergent is ammonium or suiphonate salt of long chain carboxylic acid.
- In detergent, the functional group sodium sulphonate (- SO3Na) is attached to the long chain of hydrocarbon.

HBSE 10th Class Science Carbon and Its Compounds InText Activity Questions and Answers
Textbook Page no – 61
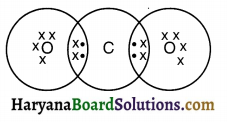
Question 1.
What would be the electron dot structure of carbon dioxide which has the formula CO2?
Answer:
The electronic configuration of carbon is (2, 4) and that of oxygen is to 6. Hence, the electron dot structure of carbon dioxide will be as shown below.
Question 2.
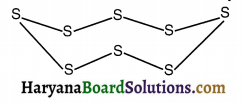
What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur?
(Hint – The eight atoms of sulphur are joined together in the form of a ring.)
Answer:
The electronic configuration of sulphur is (2, 8, 6). Hence, the electron dot structure of sulphur will be as shown below, It is a wavy structure.
Textbook Page no – 68-69
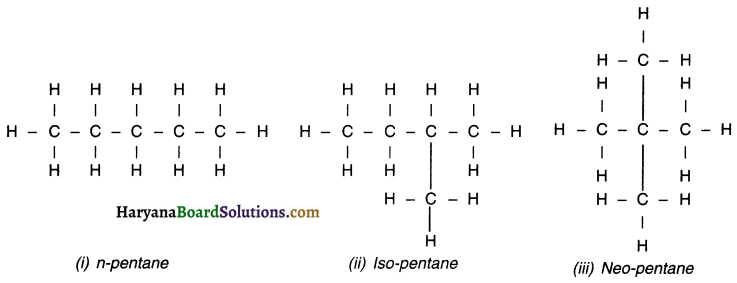
Question 1.
How many structural isomers can you draw for pentane?
Answer:
We can draw three structural isomers of pentane (C5H12). They are:
Question 2.
What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Answer:
(1) Catenation:
- Carbon has a unique ability to bond with other atoms of carbon and form long chain. This unique property of carbon is called catenation.
- Catenation results in formation of large molecules. Moreover, the ability of carbon to bond with several elements results in formation of a large number of carbon based compounds.
- Carbon atom bonds with the help of three types of covalent bonds namely, single bond, double bond and triple bond.
![]()
(2) Tetravalency:
- The valency of carbon is 4, i.e. carbon has 4 electrons in its outermost shell. Hence, carbon is called tetravalent.
- Since the valency of carbon is 4, it is capable of bonding with four other atoms of carbon or some mono-valent atoms i.e. atoms having one valency.
- This way, carbon forms compounds with oxygen, hydrogen, nitrogen, sulphur, chlorine and many other elements and gives rise to several compounds.
(3) Other reasons:
- Carbon forms very strong bonds with other elements and so the compounds formed are extremely stable.
- No other element shows the property of catenation to the extent of carbon.
Question 3.
What will be the formula and electron dot Structure of cyclopentane?
Answer:
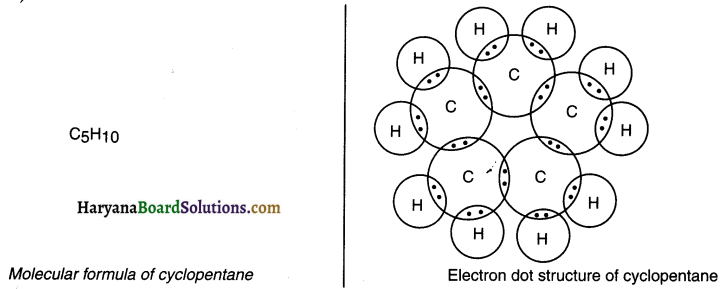
Question 4.
Draw the structures for the following compounds.
(i) Ethanoic acid
(ii) Bromopentane
(iii) Butanone
(iv) Hexanal
Are structural isomers possible for bromopentane?
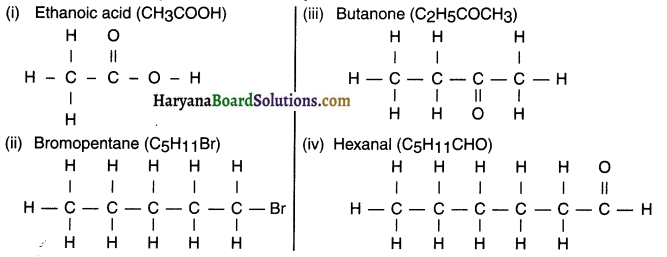
Yes, structural isomers for bromopentane are possible.
Isomers of bromopentane (C5H11Br):
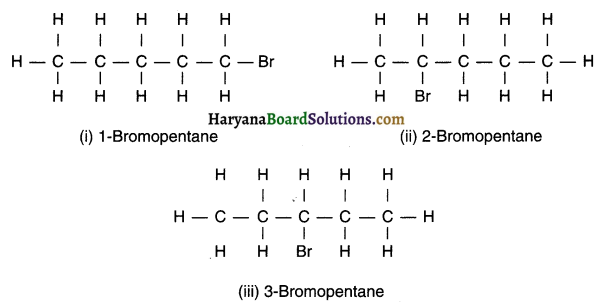
![]()
Question 5.
How would you name the following compounds?
Answer:
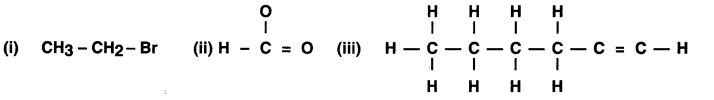
The name of the compounds are (i) Bromoethane, (ii) Methanal and (iii) 1, Hexyne
Textbook Page no – 71.
Question 1.
Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Answer:
For conversion of ethanol to ethanoic acid, oxygen is added to ethanol by oxidizing agents like alkaline potassium permanganate or acidified potassium dichromate. Hence, it is called an oxidation reaction.
Question 2.
A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Answer:
Over and above oxygen, air also contains other gaseous contents. So, sufficient oxygen will not be available for wielding and it will result in incomplete combustion with a sooty flame. Using pure oxygen will give a complete combustion with blue flame. Hence, oxygen and not air s used for welding.
Textbook Page no – 74
Question 1.
How would you distinguish experimentally between an alcohol and a carboxyllc acid?
To experimentally distinguish alcohol and a carboxylic acid we can conduct the following tests:
Answer:
(a) Acid test:
- Take few drops of alcohol (let us say ethanol) in one test tube and few drops of carboxylic acid in another test tube.
- Add sodium hydrogen carbonate to each test tube and observe.
- The test-tube which produces brisk effervescence (= quick bubbling or fizzing in solution) of CO2 gas will be carboxylic acid.
- You will see that ethanoic acid produces brisk effervescence of CO2 gas.
(b) Alcohol test:
- Take small amount of ethanol and ethanoic acid in test tube A and B respectively.
- Add 5% solution of alkaline potassium permanganate drop by drop to this solution and warm the test tubes.
- The colour of potassium permanganate will disappear in test tube containing alcohol and not in test-tube containing acid.
Question 2.
What are oxidizing agents?
Answer:
Oxidizing agents are substances which add oxygen to other substances.
Example:
Alkaline potassium permanganate or acidified potassium dichromate can give oxygen to alcohols to convert them into carboxylic acids.
Textbook Page no – 76
Question 1.
Would you be able to check if water is hard by using a detergent?
Answer:
Detergent is equally effective in both hard and soft water. Hence, we cannot check if the water is hard or soft using detergent.
![]()
Question 2.
People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture Is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Answer:
1. The dirt that sticks to our clothes is oily in nature. We know that oil is not soluble water. So, if we simply rinse the dirty cloth in water, the cloth will not become clean because the oily dirt will not dissolve in water and remain stuck to the cloth. So, to remove the dirt one has to make some action that can help to break the dirt or make it thin.
2. Agitation i.e. stirring the clothes is also one such act that helps in making the dirt layer thin. Later, the action of soap easily cleans the clothes.
Activities
Activity 1.
Students should do this activity on their own.
Activity 2.
Aim: To Study homologous series
Question 1.
Calculate the difference in the formula and molecular masses for
(a) CH3OH and C2H5OH,
(b) C2H5OH and C3H7OH, and
(c) C3H7OH and C4H9OH.
Answer:

Question 2.
Is there any similarity in these three?
Answer:
All the compounds belong to the same functional group of alcohol ( – OH). Hence, all the compounds have similar chemical properties.
Question 3.
Arrange these alcohols In the order of increasing carbon atoms to get a family. Can we call this family a homologous series?
Answer:
CH3OH, C2H5OH, C3H7OH, C4H9OH. Yes this is a homologous series of alcohols.
![]()
Question 4.
Generate the homologous serles for compounds containing up to four carbons for the other functional groups given in table 4.3 of textbook.
Answer:
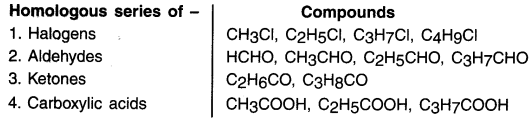
Activity 3.
Aim: To study the flame obtained by burning carbon compounds.
Caution: This activity needs the teacher’s assistance.
- Take some carbon compounds (naphthalene, camphor, alcohol) one by one on a spatula and burn them.
- Observe the nature of the flame and note whether smoke is produced.
- Place a metal plate above the flame.
- Is there a deposition on the plate in case of any of the compounds?
Answer:
The nature of flames obtained by burning various carbon compounds is given in the table below:
|
Carbon compounds |
Nature of flame | Deposits on metal plate |
| Camphor Alcohol Naphthalene |
Non-sooty flame Non-sooty flame Sooty flame |
No Carbon deposits occur |
Observation and conclusion:
1. Camphor and alcohol burns with a blue non-sooty flame without any residue. This means complete combustion takes place. As a result, alcohol is a saturated compound.
2. Napthalene burns with a yellow sooty flame leaving carbon deposits. This means napthalene undergoes incomplete combustion and is an unsaturated hydrocarbon.
Activity 4.
Aim: To study the type of flame formed by complete combustion and incomplete combustion.
Procedure:
Light a Bunsen burner.
(i) When do you get a yellow, sooty flame
(ii) When do you get a blue flame?
Answer:
(i) If some of the holes of the burner are blocked, the burner receives insufficient oxygen and hence we get a yellow sooty flame.
(ii) If all the holes of the burner are clean and open, the burner gets sufficient oxygen and the flame is blue.
Activity 5.
Aim:
1. To study the oxidation of alcohols with alkaline KMnO4.
2. Take about 3 mL of ethanol in a test tube. Warm it gently in a water bath. Add to it 5% alkaline potassium permanganate solution slowly drop by drop.
3. Does the colour of potassium permanganate persist when it is added initially?
4. Why does the colour of potassium permanganate not disappear when excess is added?
Answer:
1. No, initially the colour of potassium permanganate disappears. This happens because potassium permanganate (KMnO4) oxidizes ethanol to ethanoic acid and itself gets reduced to MnO2.
2. When excess potassium permanganate is added, the colour does not disappear because due to excess KMnO4 there is no alcohol left in the test-tube and hence no reaction takes place.
![]()
Activity 6.
Aim: To study the reaction of sodium metal with ethanol.
Answer:
Teacher’s demonstration:
Drop a small piece of sodium, about the size of a couple of grains of rice, into ethanol (absolute alcohol). What do you observe? How will you test the gas evolved?
Observation:
(i) Sodium reacts vigorously with ethanol and hydrogen gas is released.
(ii) On taking a lighted match stick near the product, the gas burns with a pop sound. This confirms that hydrogen gas is present as one of the product.
Activity 7.
Aim: To study the strength of dilute acetic acid and dilute hydrochloric acid.
1. Compare the pH of dilute acetic acid and dilute hydrochloric acid using both litmus paper and universal indicator.
2. Are both acids indicated by the litmus test?
3. Does the universal indicator show them as equally strong acids?
Answer:
1. Acetic acid as well as dilute hydrochloric acid turns litmus paper red. This confirms their acidity by the litmus test.
2. Universal indicator shows different colours for both these tests. The colour clearly tells that hydrochloric acid is stronger than acetic acid.
![]()
Activity 8.
Aim: To study the preparation of ester and its properties.
(i) Take 1 mL ethanol (absolute alcohol) and 1 mL glacial acetic acid along with a few drops of concentrated sulphuric acid in a test tube.
(ii) Warm in a water bath for at least five minutes as shown in figure.
(iii) Pour into a beaker containing 20-50 mL of water and smell the resulting mixture.
Answer:
When the mixture of absolute alcohol and glacial acetic acid is heated in the presence of concentrated sulphuric acid, it produces a sweet smelling ester called ethyl acetate.
Activity 9.
Aim: To show evolution of carbon dioxide gas when carboxylic acid reacts with sodium carbonate or sodium hydrogen carbonate.
- Take a spatula full of sodium carbonate Rubb in a test tube and add 2 mL of dilute ethanoic acid.
- What do you observe?
- Pass the gas produced through freshly el prepared lime-water. What do you observe?
- Can the gas produced by the reaction between ethanoic acid and sodium carbonate be identified by this test?
- Repeat this activity with sodium hydrogen carbonate instead of sodium carbonate.
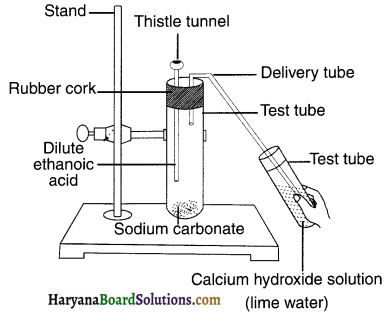
Answer:
1. A brisk effervescence appears due to the evolution of CO2 gas.
2. The gas turns lime water milky due to formation of insoluble calcium hydroxide.
3. Yes, the CO2 gas produced is identified in the test.
4. Similar observations as above will be seen when activity is done with sodium hydrogen carbonate.
Activity 10.
Aim: To study the solubility of oil in soap solution.
1. Take about lo mL of water each in two test tubes.
2. Add a drop of oil (cooking oil) to both the test tubes and label them as A and B.
3. To test tube B, add a few drops of soap solution.
4. Now shake both the test tubes vigorously for the same period of time.
5. Can you see the oil and water layers separately in both the test tubes immediately after you stop shaking them?
6. Leave the test tubes undisturbed for some time and observe. Does the oil layer separate out? In which test tube does this happen first?
Answer:
(i) No, oil and water do not separate immediately in any of the test-tubes.
(ii) Two separate layers of oil and water are formed in test-tube A while in test-tube B separate layers are not formed.
![]()
Activity 11.
Aim: To study how soap reacts with hard and soft water.
1. Take about lo mL of distilled water (or rain water) and 10 mL of hard water (from a tube well or hand-pump) in separate test tubes.
2. Add a couple of drops of soap solution to the test tubes.
3. Shake the test tubes vigorously for an equal period of time and observe the amount of foam formed.
- In which test tube do you get more foam?
- In which test tube do you observe a white curdy precipitate?
Answer:
1. The test-tube containing distilled water produces more foam.
2. The test-tube which contains hard water shows curd-like white precipitate.
Activity 12.
Aim: To study the solubility of soap and detergent in hard water.
1. Take two test tubes with about 10 mL of hard water in each.
2. Add five drops of soap solution to one and five drops of detergent solution to the other.
3. Shake both test tubes for the same period.
- Do both test tubes have the same amount of foam?
- In which test tube is a curdy solid formed?
Answer:
1. The test-tube which contains detergent solution produces more foam.
2. Curdy solid is formed in test-tube which contain soap solution.