Haryana State Board HBSE 10th Class Science Notes Chapter 2 Acids, Bases and Salts Notes.
Haryana Board 10th Class Science Notes Chapter 2 Acids, Bases and Salts
Understanding chemical Properties of Acids and Bases:
- In present times, 114 elements are known to us. These elements combine in several ways and give rise to a very large number of compounds.
- On the basis of their chemical properties all the compounds is can be divided into three groups. They are:
Acids: An acid is a compound having hydrogen which when dissolved in water releases i.e. dissociates hydrogen ions (H+) to be specific (H3O+ ions). For example, hydrochloric acid (HCl), sulphuric acid (H2SO4), etc.
Bases: A base is a metal hydroxide substance which when dissolved in water release hydroxide (OH–) ions. For example, sodium hydroxide (NaOH), calcium hydroxide (Ca(OH)2), etc.
![]()
Salts: A salt is an ionic compound which is formed from the neutralization reaction of an acid and a base.
Olfactory Indicators: Olfactory means ‘relating to the sense of smell’. Those substances whose smell changes in acidic or basic solutions are called olfactory indicators.
Outcomes of certain important types of reactions:
Reaction of acid with metal:
When acid reacts with metal, metallic salt of that metal and hydrogen gas are produced.
Reaction of base with metal:
When a strong base reacts with certain metals, it produces salt and hydrogen gas.
Reaction of acid with metal carbonate or metal hydrogen carbonate:
When acids react with metal carbonate or metal hydrogen carbonate, most acids produce salt, water and carbon dioxide gas.
Reaction of acid with base:
When acid reacts with base, salt and water are produced. Since base neutralizes the effect of acid, this reaction is called neutralization reaction.
Reaction of acid with metal oxide:
When acid reacts with metal oxide, salt and water are produced.
Reaction of nonmetallic oxide with base:
When a non-metallic oxide reacts with base, the reaction gives out salt and water.
How Strong are Acid or Base Solutions?
Strong acids:
An acid which gets completely ionized completely in water or say which completely dissociates in water and produce a large amount of hydrogen [H+] ions (or say hydronium [H2O+] ions) is called a strong acid.
![]()
Weak acids:
An acid which does not ionize completely (i.e. does not dissociate completely in water) and thus produce a small amount of hydrogen [H+] ions (or say H2O+ ions) is called a weak acid.
Strong base:
A base, which completely ionizes in water and thus produces a large amount of hydroxide (OH–) ions, is called a strong base or a strong alkali.
Weak base:
A base, which does not ionize completely in water and thus produces a small amount of hydroxide (OH–) ions, is called a weak base or a weak alkali.
The methods of measuring the strength of an acid or a base:
- Through universal indicator and
- Through pH scale
pH scale:
- The pH scale measures concentration of hydrogen [H+] ions in the solution.
- The scale points range from O to 14. 0 means very acidic and 14 means very alkaline. Scale point 7 means neutral solution.
![]()
Importance of pH in everyday life:
- Importance of pH in existence of living beings,
- Importance of pH in soil,
- Importance of pH in digestion of food,
- Importance of pH in stopping tooth decay,
- Self-defence by animals and plats through Chemical warfare:
More About Salts
Salt:
- A salt is an ionic compound which is formed by the neutralization reaction of an acid and a base. Thus, we get salt when we react an acid with a base. When we dissolve a salt in water it will get ionized and release cation (i.e. the positive +ve ion) and anion (i.e. the negative – ve ion).
- In general, salts having same type of cations (+ve ions) or anions (-ve) belong to the same family.
Some Important Salts:
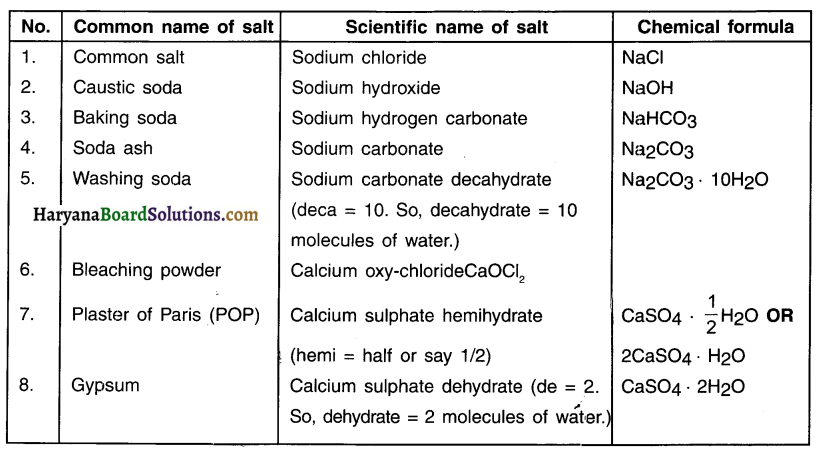
Read More: