Haryana State Board HBSE 10th Class Science Solutions Chapter 3 Metals and Non-metals Textbook Exercise Questions and Answers.
Haryana Board 10th Class Science Solutions Chapter 3 Metals and Non-metals
HBSE 10th Class Science Metals and Non-metals Textbook Questions and Answers
Question 1.
Which of the following pairs will give displacement reactions?
(a) NaCI solution and copper metal
(b) MgCI2 solution and aluminium metal
(C) FeSO4 solution and silver metal
(d) AgNO3 solution and copper metal
Answer:
(d) AgNO3 solution and copper metal
![]()
Question 2.
Which of the following methods is suitable for preventing an iron frying pan from rusting?
(a) Applying grease
(b) Applying paint
(c) Applying a coating of zinc
(d) All of these
Answer:
(c) Applying a coating of zinc
Note: Although all options can be used to prevent rusting of an ordinary iron. But, the frying pan is used for cooking so the only best option is to coat it with zinc.
Question 3.
An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble In water. The element is likely to be ……
(a) Calcium
(b) Carbon
(c) Silicon
(d) Iron
Answer:
(a) Calcium
Question 4.
Food cans are coated with tin and not with zinc because
(a) Zinc is costlier than tin.
(b) Zinc has a higher melting point than tin.
(c) Zinc Is more reactive than tin.
(d) Zinc is less reactive than tin.
Answer:
(c) Zinc Is more reactive than tin.
![]()
Question 5.
You are given a hammer, a battery, a bulb, wires and a switch.
(a) How could you use them to distinguish between samples of metals and non-metals?
(b) Assess the usefulness of these tests in distinguishing between metals and non-metals.
Answer:
1. (a) Take the given samples of metals and non-metals and strike them with the hammer. If a sample converts into a sheet, it is a metal and if not, it is non-metal. Metals are malleable, non-metals are not.
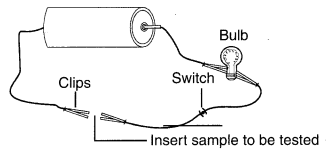
(b) If a sample produces sound when struck with the hammer, it is a metal. Metals are sonorous. But if it does not produce a round, it is non-metal.
(c) Now arranging the given objects to form an electric circuit. Insert any one sample between clips A and B, lithe bulb glows, it is a metal (good conductor of electricity). If the bulb does not glows, it is a non-metal.
2. From the above tests, it is clear that metals are generally malleable, sonorous and good conductors of electricity, while non-metals are generally non-malleable/brittle, non-sonorous and poor conductors of electricity.
Question 6.
What are amphoteric oxides? Give two examples of amphoteric oxides.
Answer:
Some metal oxides react with both acids and bases to produce salt and water. Such metal oxides are called amphoteric oxides.
Example:
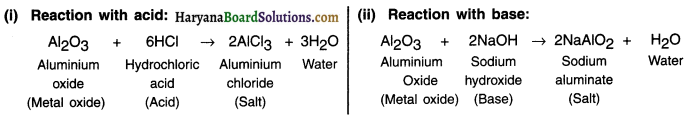
Question 7.
Name two metals which will displace hydrogen from dilute acids, and two metals which will not.
Answer:
1. Sodium and magnesium will displace hydrogen from dilute acids because they lie above hydrogen in the activity series.
2. Metals such as copper and silver lie below hydrogen in the activity series and hence will not displace hydrogen from dilute acids.
![]()
Question 8.
In the electrolytic refining of a metal M, what would you take as the anode, the cathode and the electrolyte?
Answer:
(a) Cathode (negatively charged) — Pure metal M
(b) Anode (positively charged) — Impure metal M
(c) Electroyte — Aqueous solution of a salt of metal M
Question 9.
Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in figure below.
(a) What will be the action of gas on :
(i) Dry litmus paper?
(ii) Moist litmus paper?
(b) Write a balanced chemical equation for the reaction taking place.
Answer:
(a) (i) There will be no effect of gas on dry litmus paper. Hence, no change in colour will take place in case of dry litmus paper.
(ii) Moist blue litmus paper will turn red because sulphur is non-metal and non-metal oxides are acidic in nature.
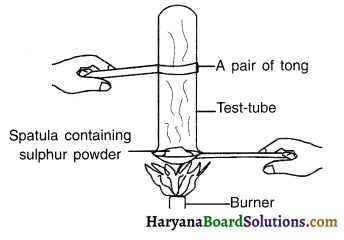
(b) (i) S + O2 → SO2
(ii) SO2 + H2O → H2SO3
![]()
Question 10.
State two ways to prevent the rusting of iron.
Answer:
Two ways to prevent rusting of iron:
(1) Paint the iron articles.
(2) Galvanize iron article with the protective layer of zinc metal.
Question 11.
What type of oxides are formed when non-metals combine with oxygen?
Answer:
When non-metals combine with oxygen they form acidic oxides. SO2, CO2, etc. are some examples.
Question 12.
Give reasons
(a) Platinum, gold and silver are used to make jewellery.
(b) Sodium, potassium and lithium are stored under oil.
(c) Aluminium is a highly reactive metal, yet it Is used to make utensils for cooking.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
Answer:
(a) Metals such as gold, platinum and silver are rare as well as non-reactive metals.
These metals have very attractive luster and so catches the attention of people. Moreover, these metals are malleable and ductile and molded into desired shape and intricated into exquisite jewellery. Another major advantage is that these metals do not corrode when exposed to air. Hence, they can be kept and worn for years without fearing the loss of luster.
(b) 1. Sodium (and potassium) is a highly reactive metal. If kept open it reacts with oxygen at room temperature. The reaction is extremely exothermic i.e. a lot of heat is produced. This even causes burning and dangerous accidents.
2. Sodium does not react with kerosene and hence it is preserved in kerosene.
(c) Using metal vessels that can react with food can lead to severe harm to the body.
Although aluminium is quite reactive, if forms a protective layer of aluminium oxide on its surface. This protects it from getting corroded and hence reacting with food. Hence, even though aluminum is very reactive metal, it is used to make utensils for cooking.
(d) It is easier and a cheaper way to reduce metal oxide to metal. Hence, carbonate and sulphide ores are first converted into their oxides and then reduced into metals.
![]()
Question 13.
You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Answer:
1. On using copper metal, it gets corroded to form copper carbonate and get tarnished i.e. copper gets decoloured due to oxidation.
2. Copper carbonate dissolves in mild acids. Lemon or tamarind juice are mild acids which when rub on copper vessels dissolve copper carbonate and make copper vessels shiny again.
Question 14.
Differentiate between metal and non-metal on the basis of their chemical properties.
Answer:
| metal | non-metal |
| Metals form basic oxides. Metals can displace hydrogen atoms from their dilute acids. |
Non-metals form neutral oxides. Since non-metals cannot react with dilute acids, they cannot replace hydrogen atoms from dilute acids. |
Question 15.
A man went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty retreat. Can
you play the detective to find out the nature of the solution he had used?
Answer:
1. The crooked goldsmith fooled her by using a specific solution. The name of this solution is aqua regia.
2. This solution contains 1 part of concentrated nitric acid (HNO3) and 3 parts of hydrochloric acid (HCl). On putting gold Ornaments in this solution, the solution dissolves gold.
Question 16.
Give reasons why copper is used to make hot water tanks and not steel (an alloy of Iron).
Answer:
1. The advantages of using copper over steel for making hot water tanks are many.
2. Copper does not react with any form of metal, not even steam.
3. Copper is cheap.
4. Copper is easily available and is also a good conductor of heat which enables heating water.
HBSE 10th Class Science Metals and Non-metals InText Activity Questions and Answers
Textbook Page no – 40
Question 1.
Give an example of a metal which
(i) is a liquid at room temperature
(ii) can be easily cut with a knife
(iii) is the best conductor of heat
(iv) is a poor conductor of heat
Answer:
(i) Mercury
(ii) Sodium
(iii) Silver
(iv) Lead
![]()
Question 2.
Explain the meanings of malleable and ductile.
Answer:
1. Some metals can be hammered and turned into thin sheets. This property of the metals is known as malleability.
2. This property is specially found in metals like gold, silver and aluminium. Hence, thin strips can be prepared from gold and silver and very thin paper-like foil can be prepared from aluminium.
3. Some metals are ductile i.e. we can draw thin wires from them. For e.g. gold and silver.
4. For example, about 2 kilometer long wire can be drawn from one gram gold. Wires can also be prepared from metals like copper and aluminium, by drawing.
Textbook Page no – 46
Question 1.
Why Is sodium kept Immersed in kerosene oil?
Answer:
1. Sodium (and potassium) is a highly reactive metal. If kept open it reacts with oxygen at room temperature, The reaction is extremely exothermic i.e. a lot of heat is produced. This even causes burning and dangerous accidents.
2. Sodium does not react with kerosene and hence it is preserved in kerosene.
Question 2.
Write equations for the reactions of ……….
(i) Iron with steam,
(ii) Calcium and potassium with water
Answer:
Question 3.
Samples of four metals A, B, C and D were taken and added to the following solution one by one. The results obtained have been tabulated as follows.
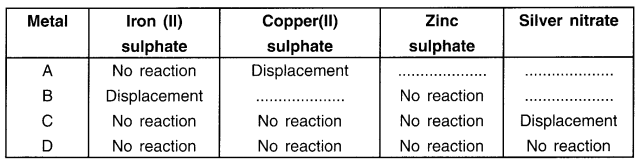
Use the table above to answer the following questions about metals A, B, C and D.
(i) Which Is the most reactive metal?
(ii) What would you observe if B is added to a solution of copper (Il) sulphate?
(iii) Arrange the metals A, B, C and D in the order of decreasing reactivity.
Answer:
(i) B is the most reactive metal because it displaces iron from its salt solution.
(ii) On adding ‘B’ to copper sulphate solution, blue colour of copper sulphate solution disappears and reddish brown coloured copper metal gets deposited on metal B. This happens because B will displace Cu from CuSO4.
(iii) The order of reactivity is B > A > C > D.
![]()
Question 4.
Which gas is produced when dilute hydrochloric acid is added to a reactive metal? Write the chemical reaction when iron reacts with dilute H2SO4 :
Answer:
Hydrogen gas is produced when dilute hydrochloride acid is added to a reactive metal.
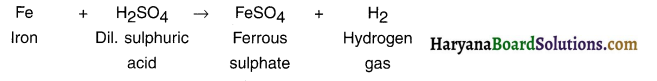
Question 5.
What would you observe when zinc Is added to a solution of iron(lI) sulphate? Write the chemical reaction that takes place.
Answer:
Zinc is more reactive than iron. Hence, when zinc is added to iron (II) sulphate, zinc displaces iron metal and the colour of solution fades from green to colourless.

Textbook Page no – 49
(i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of Na2O and MgO by the transfer of electrons.
(iii) What are the ions present in these compounds?
Answer:
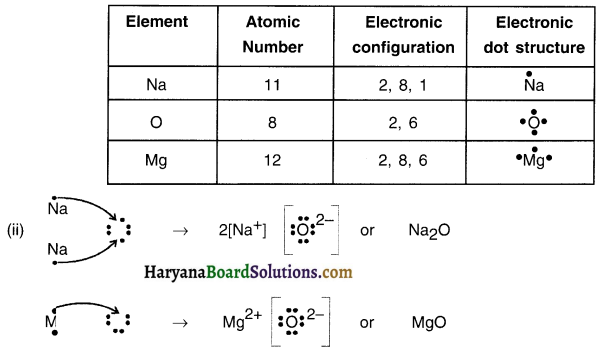
(iii) In Na2O ions present are Na+ and O2- — In MgO, ions present are Mg2 and
Question 2.
Why do ionic compounds have high melting points?
Answer:
Melting point and boiling point :
In ionic compounds, positive and negative ions are joined strongly due to inter-ionic attraction. As a result, more energy is required to break such compounds and hence their melting and boiling points are high.
Textbook Page no – 53
Question 1.
Define the following terms.
(i) Mineral (ii) Ore (iii) Gangue
Answer:
(i) Mineral:
The elements or compounds that occur naturally in the earth’s crust are called minerals. Sea-water also contains salts of metals such as sodium chloride, magnesium chloride, etc.
![]()
(ii) Ores:
Those minerals from which the metals can be extracted, conveniently and profitably are called ores.
(Note: Although all minerals contain metals, they are not termed as ‘ores’. The reason behind this is that is not always practically possible to extract metals from these minerals. Hence, only those minerals in which metals is present in large quantity and from which metals can be extracted conveniently and reasonably are called ores.)
(iii) Gangue:
Impurities such as sand, mud, etc. present in the ore are called gangue.
Question 2.
Name two metals which are found in nature in the free state.
Answer:
Gold and silver.
Question 3.
What chemical process is used for obtaining a metal from 4ts oxide?
Answer:
Metal oxides can be converted to metal through the process of reduction using carbon (coke).
Example: Zinc oxide is reduced to obtain zinc metal by heating it with carbon.
ZnO(s) + C(s) → Zn(s) + CO(s)
Over and above carbon, highly reactive metals such as sodium, calcium, aluminium are also used as reducing agents.
Example: Fe2O3(s) + 2Al(s) → 2Fe(1) + Al2O3(s) + Heat
Textbook Page no – 55
Question 1.
Metallic oxides of zinc, magnesium and copper were heated with the following metals.
| Metal | Zinc | Magnesium | Copper |
| Zinc oxide | |||
| Magnesium oxide | |||
| Copper oxide |
In which cases will you find displacement reactions taking place?
Answer:
| Metal | Zinc | Magnesium | Copper |
| Zinc oxide | No reaction | Displacement | No reaction |
| Magnesium oxide | No reaction | No reaction | No reaction |
| Copper oxide | Displacement | Displacement | No reaction |
Answer:
As per the reactivity series, magnesium is most reactive, then comes zinc and finally copper i.e. Mg > Zn > Cu. So, in the first column, zinc cannot displace itself. It also cannot displace magnesium because magnesium is more reactive than zinc. However, since zinc is more reactive than copper, it can displace copper. Similar events happen in the next two columns.
![]()
Question 2.
Which metals do not corrode easily?
Answer:
Gold and platinum
Question 3.
What are alloys?
Answer:
An alloy is a homogeneous mixture of two or more metals or metal and non-metal.
Activities
Activity 1.
Perform an activity to study that metals are lustrous.
Answer:
Take samples of iron, copper, aluminium and magnesium. Note the appearance of each sample.
1. Clean the surface of each sample by rubbing them with sand paper. Note their appearance again.
2. Initially the surface of these metals look dull. On rubbing with sand paper, the surface starts shinning This happened because pure metals have shiny surface.
Activity 2.
Perform an activity to study that metals are hard.
Answer.
1. Take small pieces of iron, copper, aluminium and magnesium. Try to cut these metals with a sharp knife. What do you observe?
2. Hold a piece of sodium metal with a pair of tongs. (Caution: Always handle sodium metal with care.) Dry it by pressing between the folds of a filter paper. Now, put it on a watch-glass and try to cut it with a knife. What do you observe?
Observation:
1. Pieces of iron, copper and aluminium are very hard and hence it is difficult to cut them with a knife. However, magnesium turned out to be soft and so could be cut
2. We can see that sodium metal gets cut easily with the knife.
Activity 3.
Perform an activity to study that metals are malleable.
1. Take pieces of iron, zinc, lead and copper.
2. Place any one metal on a block of iron and strike it four or five times with a hammer. What do you observe?
3. Repeat with other metals and record the change in the shape of these metals.
Answer:
1. On striking hammer, the metal becomes flat. This means metal is malleable on hammering.
2. Pieces of iron, zinc, lead and copper also become flat i.e. get converted into sheets on hammering.
3. This property of metals under which they can be hammered and made flat is called the property of malleability.
![]()
Activity 4.
Perform an activity to study that metals are ductile. Consider some metals such as iron, copper, aluminium, lead, etc. Which of these metals are also available in the form of wires?
Answer:
All the four metals mentioned here are available in the form of wire also. This property of metals where in they can be drawn into wires is called the property of ductility.
Activity 5.
Perform an activity to study that metals are good conductors of heat.
Answer:
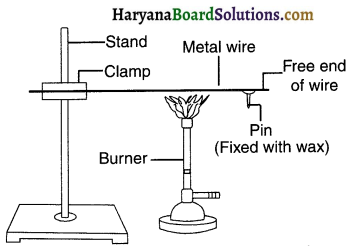
1. Take an aluminium or copper wire. Clamp this wire on a stand, as shown in figure 1.
2. Fix a pin to the free end of the wire using wax.
3. Heat the wire with a spirit lamp, candle or a burner near the place where it is, clamped.
Activity (i).
What do you observe after sometime? Does the metal melt?
Answer:
1. On heating the wire on one end, soon the entire wire gets hot. The heat melts the wax and so the pin attached to it falls down. This means the heat given at one end of the metal got transferred to the entire metal wire. So, we conclude that aluminium and copper are good conductors of heat.
2. The heat melted the wax but not the metal wire because the temperature of the heat is not high enough.
Activity 6.
Perform an activity to study that metals are good conductors of electricity.
Set up an electric circuit as shown in figure.
Place the metal to be tested in the circuit between terminals A and B as shown. Does the bulb glow? What does this indicate?
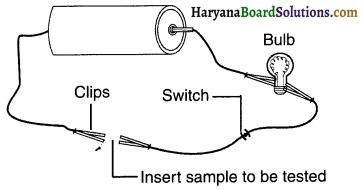
Answer:
On joining the metal such as iron, aluminium or copper with two terminals namely A and B, the bulb starts glowing. This means that electricity flows through the metals. Hence, metals are good conductor of electricity.
![]()
Activity 7.
Aim: To study the properties of non-metals
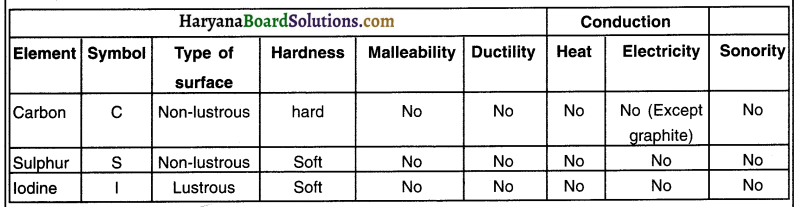
Collect samples of carbon (coal or graphite), sulphur and iodine.
Question 1.
Carry out activities 1 to 6 with these non-metals and record your observations.
Answer:
Samples of carbon, sulphur and iodine do not show the properties of metals. However, carbon in the form of graphite is a good conductor of electricity and iodine has a shining surface.
Activity 8.
Perform an activity to study if the oxides of magnesium and sulphur are basic or acidic.
Answer:
Take a magnesium ribbon and some sulphur powder.
1. Burn the magnesium ribbon. Collect the ashes formed and dissolve them in water.
2. Test the resultant solution with both red and blue litmus paper. Is the product formed on burning magnesium acidic or basic?
3. Now burn sulphur powder. Place a test tube over the burning sulphur to collect the fumes produced. Add some water to the above test tube and shake. Test this solution with blue and red litmus paper.
Is the product formed on burning sulphur acidic or basic?
Q. Can you write equations for these reactions?
Answer:
On burning magnesium, magnesium oxide (MgO) is formed. The solution of magnesium oxide turns red litmus paper into blue. This means that magnesium oxide is basic.
![]()
1. When sulphur powder is burnt it produces sulphur dioxide (SO2). Solution of SO2 turns blue litmus into red. Hence, SO2 is acidic.
The reactions of both these processes are as follows:
(i) 2Mg(s)+ O2(g) → 2MgO(5)
(ii) S(s) + O2(g) → SO2(g)
Conclusion:
Oxide of magnesium is basic whereas oxide of sulphur is acidic.
Activity 9.
Aim: To study how metals burn in air
Caution: The following activity needs the teacher’s assistance. It would be better if students wear eye protection.
1. Collect samples of the following metals — Aluminium, copper, iron, lead magnesium, zinc and sodium.
2. Hold any of these samples with a pair of tongs and try burning over a flame. Repeat with the other metal samples.
3. Collect the product, if formed.
4. Let the products and the metal surface cool down.
Question 1.
Which metals burn easily?
Answer:
Sodium, potassium and magnesium burn easily.
Question 2.
What flame colour did you observe when the metal burnt?
Answer:
Refer table given below.
Question 3.
How does the metal surface appear after burning?
Answer:
Refer table given below.
Question 4.
Arrange the metals In the decreasing order of their reactivity towards oxygen.
Answer:
The reactivity of metals with oxygen decreasing order is –
Na > Ca > Mg > Al> Zn > Fe > Cu
![]()
Question 5.
Are the products soluble in water?
Answer:
Refer table given below.
|
Sr. no. |
Mental | Colour of flame | Appearance of metal surface | Solubility In water |
| 1.
2. 3. 4. 5. 6. 7. |
Cu
Fe Na Mg Ca Zn AI |
Green-blue
No colour Yellow White light Brick red colour No colour White flame |
Black
Reddish White White White White White |
Insoluble Insoluble Soluble Soluble in hot water Partially soluble Insoluble Insoluble |
Activity 10.
Aim: To study how metals react with water.
Answer:
Caution: This activity needs the teacher’s assistance.
1. Collect samples of the following metals — Aluminium, copper, iron, lead magnesium, zinc, calcium, sodium
and potassium.
2. Put small pieces of the samples separately in beakers half-filled with cold water.
Question 1.
Which metals reacted with cold water? Arrange them in the increasing order of their reactivity with cold water.
Answer:
Na, K and Ca reacted with cold water. The reactivity of metals with cold water in increasing order is: Mg < Ca < Na < K
Question 2.
Did any metal produce fire on water?
Answer:
Sodium (Na) and potassium (K) catch fire on water.
![]()
Question 3.
Does any metal start floating after some time?
Answer:
Calcium and magnesium start floating after some time.
1. Put the metals that did not react with cold water in beakers half-filled with hot water.
2. For the metals that did not react with hot water, arrange the apparatus as shown in the figure and observe their reaction with steam.
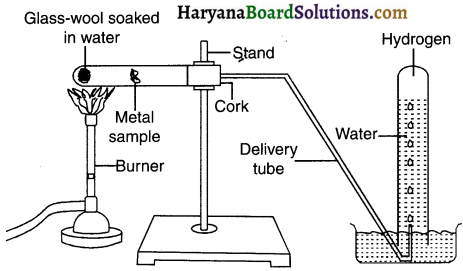
Question 4.
Which metals did not react even with steam?
Answer:
Metals namely copper, silver and gold did not react even with steam.
Question 5.
Arrange the metals, In the decreasing order of reactivity with water.
Answer:
Metals such as lead (Pb), copper (Cu), silver (Ag) and gold (Au) did not react with water. The reactivity of remaining metals with water in decreasing order is: K> Na > Ca > Mg > Al> Zn > Fe
Activity 11
Aim: To study how metals react with acids.
Answer:
Collect the sample of metal pieces such as magnesium, alumimnium, zinc, iron and copper. If the samples are tarnished, rub them clean with sand paper.
Caution: Do not take sodium and potassium as they react vigorously even with cold water.
1. Put the samples separately in test tubes containing dilute hydrochloric acid.
2. Suspend thermometers in the test tubes, so that their bulbs are dipped in the acid.
Question 1.
Observe the rate of formation of bubbles carefully.
Answer:
The rate of formation of bubbles is fastest in magnesium.
Question 2.
With which metal did you record the highest temperature?
Answer:
With magnesium (Mg).
Question 3.
Which metals reacted vigorously with dilute hydrochloric acid?
Answer:
Metals like Mg, Al, Zn and Fe react vigorously with dilute hydrochloric acid.
Question 4.
Arrange the metals in the decreasing order of reactivity with dilute acids.
Answer:
Mg > AI > Zn> Fe
![]()
Activity 12.
Aim: To study the reaction of metals with solution of other metals.
Answer:
1. Take a clean piece of copper wire of copper and an iron nail.
2. Put the copper wire in a solution of iron sulphate and the iron nail in a solution of copper sulphate taken in test tubes
3. Record your observations after 20 minutes.
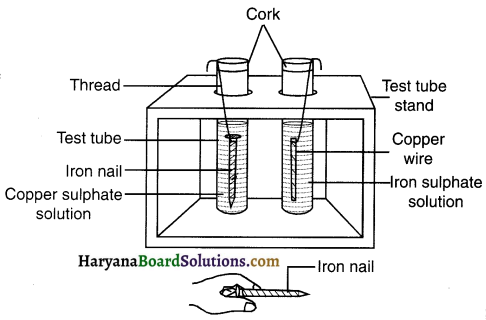
Question 1.
In which test tube did you find that a reaction has occurred?
Answer:
The reaction took place in the test tube in which the iron nail is kept in copper sulphate (CuSO4) solution.
Question 2.
On what basis can you say that a reaction has actually taken place?
Answer:
The blue colour of the copper sulphate solution starts fading. This assures occurrence of some chemical reaction.
Question 3.
Can you correlate your observations for activities 3.9, 3.10 and 3.11?
Answer:
This activity can be correlated with activities 3.9, 3.10 and 3.11 in the sense that all these activities affirm that iron (Fe) is more reactive than copper (Cu).
Question 4.
Write a balanced chemical equation for the reaction that has taken place.
Answer:
Chemical reaction:
Fe(s) + CUSO4(aq) → CU(g) + FeSO4(aq)
Question 5.
Name the type of reaction.
Answer:
The reaction is displacement reaction.
![]()
Activity 13.
Aim: To study the properties of ionic compounds.
Take samples of sodium chloride, potassium iodide, barium chloride or any other salt from the science laboratory.
1. What is the physical state of these salts?
Take a small amount of a sample on a metal spatula and heat directly on the flame. Repeat with other samples.
2. What did you observe? Did the samples impart any colour to the flame? Do these compounds melt?
3. Try to dissolve the samples in water, petrol and kerosene. Are they soluble?
4. Make a circuit as shown in figure and insert the electrodes into a solution of one salt. What did you observe? Test the other salt samples too in this manner.
5. What is your inference about the nature of these compounds?
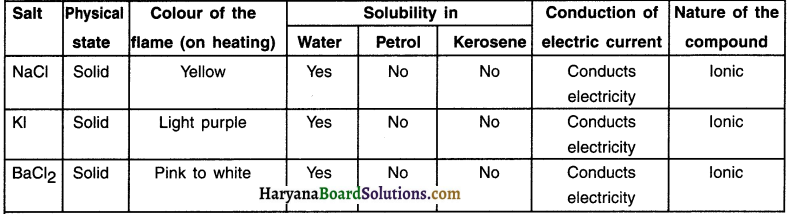
Answer:
The answers of all these questions are tablulated below:
Activity 14.
Perform an activity to demonstrate that both air and water are needed for rusting of Iron.
Answer:
1. Take three test tubes and place clean iron nails in each of them.
2. Label these test tubes A, B and C.
3. Pour some water in test tube A and cork it. The nail should not completely submerge.
4. Pour boiled distilled water in test tube B, add about 1 mL of oil and cork it. The oil will float on water and prevent the air from dissolving in the water.
5. Put some anhydrous calcium chloride in test tube C and cork it. Anhydrous calcium chloride will absorb the moisture, if any, from the air. Leave these test tubes for a few days and then observe.
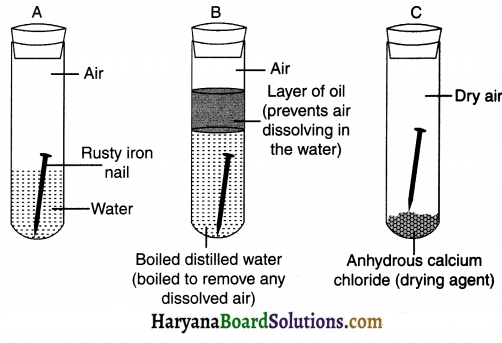
Observation:
- Nail in test tube A is in contact with water as well as air and so it will get rusted.
- Nail in test tube B is in contact with water but not air and so it will not rust.
- Nail in test tube C is in contact with air but not water and so it will not rust.
![]()
Conclusion :
- The nail in test-tube A got rusted due to oxidation reaction.
- The activity proves that rusting of iron can take place only if iron is in contact with (1) water as well as (2) air.