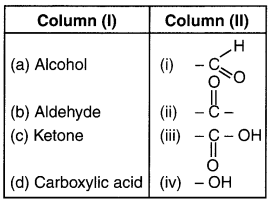
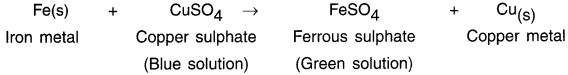
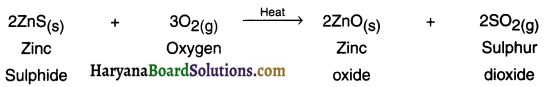
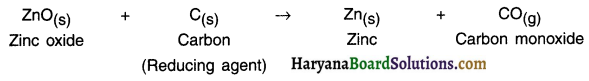
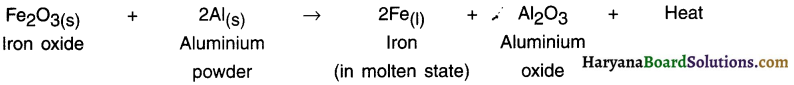
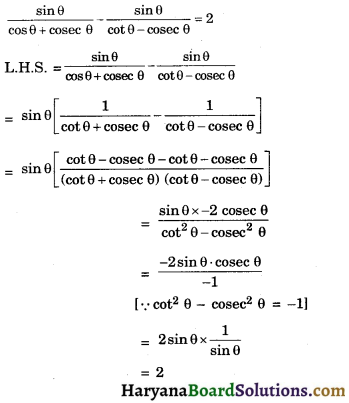
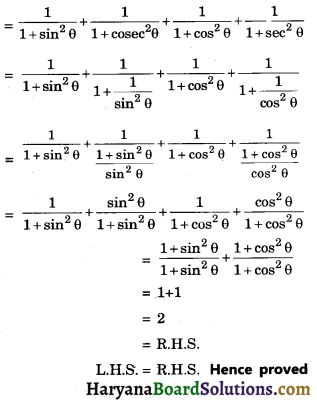
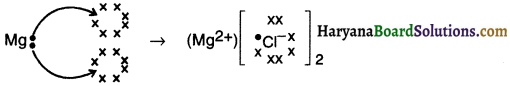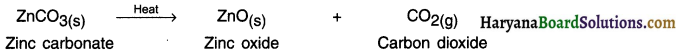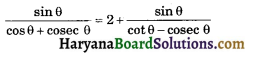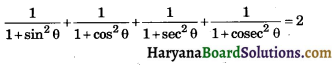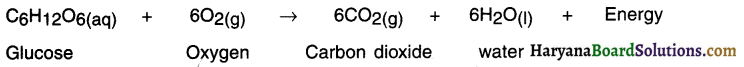HBSE 10th Class Science Important Questions Chapter 6 Life Processes
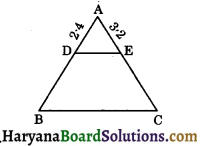
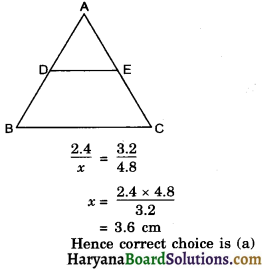
Haryana State Board HBSE 10th Class Science Important Questions Chapter 6 Life Processes Important Questions and Answers.
Haryana Board 10th Class Science Important Questions Chapter 6 Life Processes
Question 1.
Only what is visible is alive does not hold true for a life. Explain.
Answer:
1. When we see an animal moving we call it alive. Similarly when we see a green plant we call it alive.
2. Actions that we can see such as running, breathing, laughing, changing colours of leaves, etc. also make us believe that the organism is alive.
3. So, we believe some sort of movement, either growth-related or not as an evidence of being alive.
4. But, a plant which we cannot see growing and animals that breathe without showing any movement are also alive. Hence, it would be wrong to say that what is visible is only alive.
![]()
Question 2.
How one can differentiate living organisms and non-living things? What are the exceptions here?
Answer:
1. Movement is one of the basic criterions to identify life. All living organisms show movement without any external help.
2. Animals show clear visible movements.
3. Plants show invisible movements inside their body (i.e., movements of various elements and compounds). External movement of plants is quite minute and very slow and so it is difficult to notice.
4. Viruses are an exception here.
Question 3.
What do you mean by molecular movement? Explain.
Answer:
1. The movements of molecules within living organisms which we cannot see through our naked eyes but are critical for carrying life processes such as photosynthesis, respiration, digestion, excretion, etc. are called molecular movements.
2. Molecular movement includes movement of molecules such as oxygen for respiration, movement of compounds such as enzymes, nutrients, hormones, etc.
Need of molecular movement:
- Living organisms are highly complex, but very well organized structures. Going down they are made of organs, tissues, cells and cell organelles.
- Therefore it is necessary that molecules move and reach up to cell level so that functions such as growth, maintenance and survival are maintained.
- For example, oxygen inhaled move throughout the body via, the process of breathing.
Question 4.
What is the connecting link between living organisms and non-living things? Why?
Answer:
Viruses are the connecting link between living organisms and non-living things.
Reason:
1. It is said that molecular movement is necessary in living organisms. Non-living things do not show any molecular movement.
2. In a free environment, viruses do not show any molecular movement. But, when they come in the contact of the host cells they start acting as living organisms in the form of obligate parasite.
3. Thus, viruses on one side do not show molecular movement i.e. behave as non-living things, but under favourable environment they start showing molecular movement and behave like living organisms. Hence, viruses are called a connecting link between living organisms and non-living things.
![]()
Question 5.
What are life processes? State the important life processes that perform the job of maintaining these processes.
Answer:
Life processes:
1. All living organisms perform certain important functions to maintain their survival. These main functions are called life processes.
2. In other words, the processes which together perform the job of maintenance of the body are known as life processes.
3. Maintenance process requires energy. The energy is used for—
- Growth and maintenance of organisms and
- Preventing break-down.
The energy needed to the organism comes from external sources such as food, sun, atmosphere, etc.
The main life processes are:
(1) Nutrition,
(2) Respiration,
(3) Transportation,
(4) Excretion,
(5) Control and co-ordination,
(6) Movement and
(7) Reproduction.
Question 6.
Define and explain each life process very briefly.
Answer:
1. Nutrition:
- The process of transferring a source of energy which we call food, from outside the body of the organism to the inside is called nutrition.
- The food consumed is then converted into smaller and smaller units so that it can be absorbed by the body.
- Most of the food sources are carbon-based. Depending upon the complexity of these carbon sources, different organisms can then use different kind of nutritional processes.
2. Respiration:
- The process of acquiring oxygen from outside the body, and it in the process of break-down of food-sources for cellular needs, is known as respiration.
- Through respiration, organisms break down food and release energy.
3. Transportation:
- The process through which absorbed substances are transported to various parts of the body is called transportation.
- Transportation system is important to carry food and oxygen from one place to another.
- This system is also important for carrying waste products from body cells to excretory organs.
![]()
4. Excretion:
Waste materials produced in various cells of the body are removed by the process of excretion.
5. Control and co-ordination (Chapter 7):
Control and co-ordination make the living organisms adapt to the changing environment and survive there in.
6. Movement (Chapter 7):
The process of movement makes the living organism move from one place to another.
7. Reproduction (Chapter 8):
Reproduction involves multiplication of existing organisms. This enables them to maintain the existence of their species on the earth.)
Question 7.
What are nutrients? Give examples.
Answer:
1. The substances obtained by an organism from the surroundings and used them as a source of energy are called nutrients.
2. For example, carbohydrates, lipids, proteins, mineral, salts, etc.
Question 8.
Why a unicellular organism does not need specific organs for nutrition, respiration and transportation?
Answer:
In unicellular organisms, entire body surface of the organism is in the direct contact with the environment and transfer can take place through entire body. So, a unicellular organism does not need specific organs for nutrition, respiration and transportation.
Question 9.
What is nutrition? Classify the types of nutrition.
Answer:
1. Nutrition:
- The process of transferring a source of energy which we call food, from outside the body of the organism to the inside is called nutrition.
- The food consumed is then converted into smaller and smaller units so that it can be absorbed by the body.
- Most of the food sources are carbon-based. Depending upon the complexity of these carbon sources, different organisms can then use different kind of nutritional processes.
2. Respiration:
- The process of acquiring oxygen from outside the body, and it in the process of break-down of food-sources for cellular needs, is known as respiration.
- Through respiration, organisms break down food and release energy.
3. Transportation:
- The process through which absorbed substances are transported to various parts of the body is called transportation.
- Transportation system is important to carry food and oxygen from one place to another.
- This system is also important for carrying waste products from body cells to excretory organs.
4. Excretion:
Waste materials produced in various cells of the body are removed by the process of excretion.
5. Control and co-ordination (Chapter 7):
Control and co-ordination make the living organisms adapt to the changing environment and survive there in.
6. Movement (Chapter 7):
The process of movement makes the living organism move from one place to another.
![]()
7. Reproduction (Chapter 8):
Reproduction involves multiplication of existing organisms. This enables them to maintain the existence of their species on the earth.)
Types of nutrition:
(1) Autotrophic nutrition,
(2) Heterotrophic nutrition:
- Saprophytic nutrition
- Parasitic nutrition
- Holozoic nutrition
Question 10.
Define autotrophic nutrition and give examples of autotrophes.
Answer:
Autotrophic nutrition:
1. ‘Auto’ means ‘Self’ and ‘Trophe’ means ‘Nutrition’. So, the mode of ‘nutrition’ in which the organism ‘itself’ synthesizes its own food from the simple inorganic materials like carbon dioxide and water with the help of sunÍight, is called autotrophic nutrition.
2. All green plants and some bacteria show autotrophic mode of nutrition.
Question 11.
How do autotrophs fulfill their need of carbon and energy?
Answer:
1. Carbon dioxide (CO2), (2) Water (H2O) and (3) Nutrients are the main food materials that the autotrophic organisms need for their growth and maintenance. These materials fulfill the carbon and energy requirement of the autotrophs.
2. Autotrophs obtain these materials through the process called photosynthesis.
3. During photosynthesis plants take up carbon dioxide from the atmosphere, water from the ground and in the presence of sunlight convert these materials into carbohydrates. The carbohydrate then provides energy to the plants.
4. The excess carbohydrate remains stored as starch within the plants.
5. Autotrophs fulfill their needs of minerals such as nitrogen, phosphorus, iron and magnesium from the soil.
Question 12.
Write a note on photosynthesis. OR Define photosynthesis and enlist Its main events.
Answer:
Photosynthesis:
- The process by which the green plants make their own food by converting carbon dioxide and water into carbohydrates in the presence of sunlight and chlorophyll is called photosynthesis.
- Thus, the basic materials used in photosynthesis are carbon dioxide and water.
Chemical equation of photosynthesis:
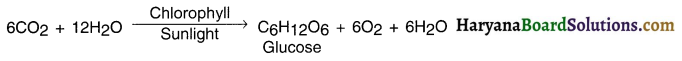
Following events take place during photosynthesis:
(1) Absorption of light energy by chlorophyll.
(2) Conversion of light energy to chemical energy and splitting of water molecule into hydrogen and oxygen.
(3) Reduction of carbon dioxide to carbohydrate.
![]()
Question 13.
Is it necessary that all events of photosynthesis take place one after the other Immediately?
Answer:
Give an example to explain.
No, it is not necessary that all the events of photosynthesis occur one after the other.
Example:
1. The desert plants absorb CO2 at night from the surrounding and repare the intermediate compound before the sun rises.
2. This intermediate compound is then acted upon after the chlorophyll absorbs solar radiation in the morning.
Question 14.
How do plants obtain carbon dioxide (CO2) from the atmosphere?
Answer:
A large number of pores are present on the leaves of plants. These pores are known as stomata. These stomata are responsible for gaseous exchange.
1. During the gaseous exchange, a large amount of water is lost. To control the water loss, the stomata is surrounded by guard cells.
2. The guard cells control the opening and closing of the stomatal pore according to the need of CO2 in the plant.
3. As the need of CO2 arises, the guard cells absorbs water and so due to turgidity (swelling up) the pores get opened.
4. When the need gets satisfied, the guard cells lose the water. So, they tend to shrink. As a result, the pores are closed.
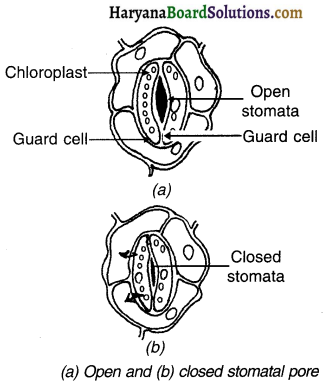
Question 15.
Draw a labeled diagram of cross-section of a leaf.
Answer:
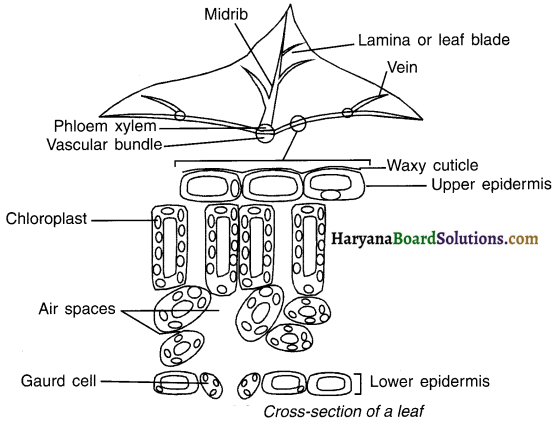
![]()
Question 16.
How do plants obtain water (H2O)?
Answer:
1. Roots of plants have specialized system to absorb underground or land water.
2. The water which is absorbed by roots reaches to the organs of the plants through xylem tissue.
3. In addition to this, the entire surface of plants is also able to absorb water Generally, plants absorb water through a process called osmosis.
Question 17.
State important functions performed by roots of terrestrial plants.
Answer:
1. In terrestnal plants, the root plays a crucial role of absorbing water and salts dissolved in the land water.
2. The root system also absorbs salts of nitrogen, phosphorus iron, magnesium, etc. from the soil.
Question 18.
What is the importance of nitrogen in plants? In which form do plants absorb nitrogen?
Answer
1. In plants, nitrogen is important for protein synthesis as well as for the production of other important substances.
2. Generally, plants absorb nitrogen in form of nitrates [N0O3] or nitrite [NO2] compounds.
3. Sometimes, the plants also take up atmospheric nitrogen prepared by symbiotic bacteria, in the form of organic compounds.
Question 19.
How is chlorophyll important for photosynthesis?
Answer:
1. Photosynthesis occurs in the chloroplast of a plant cell.
2. The chloroplast contains a pigment called chlorophyll to trap light energy.
3. The light energy of the sun is absorbed by the chlorophyll in the form of photons.
Question 20.
Define heterotrophic nutrition, and explain it.
Answer:
1. The term, ‘Hetero’ means ‘others’ and ‘Trophe’ means ‘nutrition’.
2. The mode of nutrition in which the living organisms cannot synthesis their own food material from the simple inorganic material they have and hence have to depend on other organisms for food is known as heterotrophic nutrition.
Question 21.
Describe the types of heterotrophic organisms on the basis of the type of food they consume.
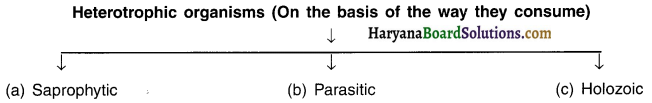
Answer:
(a) Herbivores: These organisms feed on plants.
– Generally, the food source for organisms is stationary. For example, grass and plants. Herbivores such as cow and goat have to go to these sources to obtain nutrition.
(b) Carnivores: These organisms feed on other animals.
- The food source is mobile. For example, a lion has to hunt deer which is a moving source of nutrition.
Example: Frog, tiger, lizard, etc.
(c) Omnivores: These organisms feed on plants as well as animals.
Example: human beings, dog, ant, etc.
(d) Scavengers: These organisms feed on dead materials.
Example: Vulture
Question 22.
Describe the type of heterotrophic organisms on the basis of the mode of feeding. OR Discuss the strategies used to take up the nutrition.
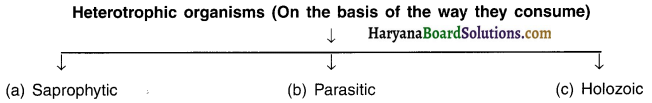
(a) Saprophytic nutrition:
- Some organisms break down the complex food material outside their bodies and then absorb it. Such a mode of nutrition is called saprophytic nutrition.
- Saprophytes absorb soluble organic nutrients from the dead parts of the animals, plants, dung, etc.
Example: Bacteria, fungi, yeast, mushrooms.
(b) Holozoic nutrition:
Some organisms undertake either a part or whole of animals or plants and then break down such sources inside their bodies. Such nutrition is known as holozoic nutrition. Example: Human beings, dog.
(c) Parasitic nutrition:
The organisms that live inside or outside of other organisms and obtain nutrition from them are known as parasites. The mode of their nutrition is called parasitic nutrition.
Example: Several bacteria, lice, tape warm, ascaris, cuscuta (plant).
Parasitic organisms derive nutrition from plants or animals without killing them.
![]()
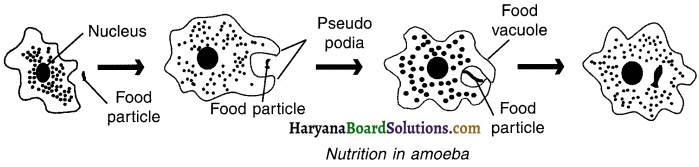
Question 23.
Describe the process of nutrition in amoeba with the help of a figure.
Answer:
Amoeba is a unicellular organism. Its mode of nutrition is holozoic.
The various processes involved in nutrition are as follows :
(a) Ingestion: When amoeba comes in contact with food particle, it ingests the food by forming temporary finger-like extensions of the cell surface called pseudopodia.
- The food is then encaptured into a bag called food vacuole.
(b) Digestion: The complex food particles are broken down into simpler forms and digested in food vacuole with the help of digestive enzymes.
(c) Absorption: The digested food is absorbed directly from food vacuole into cytoplasm by the process of diffusion.
(d) Egestion: The undigested food is moved to the surface of the cell and thrown out.
Question 24.
Differentiate between autotrophic and heterotrophic nutrition.
Answer:
Autotrophic nutrition | Heterotrophic nutrition |
| 1. In autotrophic nutrition, organisms produce their own food using water, carbon dioxide and sun light. | In heterotrophic nutrition, organisms derive energy by digesting organic substances obtained from plants and animals. |
| 2. In this nutrition, organisms obtain energy by producing carbohydrates with the help of carbon dioxide, water and sunlight. | In this nutrition, the organisms first eat the food, then digest it into simpler forms and finally obtain energy. |
| 3. Autotrophic nutrition has no further classification. | Heterotrophic nutrition can be classified into (A) Saprophytic, (B) Parasitic and (C) Holozoic nutrition. |
| 4. Example: All green plants and some bacteria. | Example: Herbivores, carnivores and omnivores. |
Question 25.
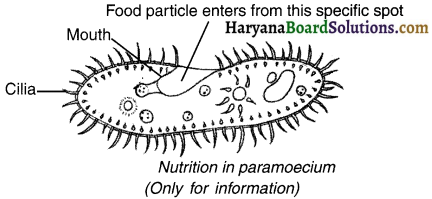
Describe the process of nutrition In paramoecium.
Answer:
1. Paramoecium is a unicellular organism whose cell has a definite shape. There is a specific spot in its cell. The entire body is covered with cilia which helps in bring the food to this specific spot.
2. The ingested (= entered) food material is then digested by paramoecium.
![]()
Question 26.
Differentiate between saprophytic and parasitic nutrition.
Answer:
| Saprophytic nutrition | Parasitic nutrition |
| The nutrition in which organisms feed on dead and decaying organic materials is known as saprophytic nutrition. | The nutrition in which organisms depend on other living organisms for their nutrition is called parasitic nutrition. |
| In this nutrition, organisms depend on dead materials. | In this nutrition, organisms depend on living organisms. |
| Unlike parasitic nutrition, there does not exist any relation between a saprophyte and the dead materials on which it feeds. | There exists a relation of a host and a parasite. |
| Example: Bacteria and fungi. | Example: Bacteria, fungi, cuscuta plant, etc. |
Question 27.
Define digestion. What are the main functions of the human digestive system?
Answer:
In animals, the process of breaking-down the large and complex food material into small and simple absorbable molecules is known as digestion. The main functions of human digestive system are:
- Ingestion: To take in food
- Digestion: Converting food into small, simple and absorbable molecules
- Absorption: To absorb the digested food
- Egestion: To remove the undigested food from the body
Question 28.
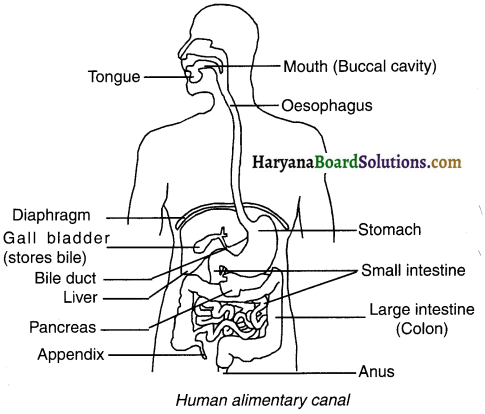
Draw a labeled diagram of human digestive system and list out the main parts associated with the process of digestion.
Answer:
Human digestive system:
1. The function of digestive system is to break down larger molecules of food into smaller forms so that they can be absorbed easily into the body.
2. The human digestive system consists of alimentary canal and its associated glands.
The main organs of the human digestive system are:
Mouth, oesophagus, stomach, small intestine and large intestine.
The main glands associated with digestion are:
Salivary gland, liver and pancreas.
Question 29.
Explain the process of digestion in humans.
Answer:
Process of digestion In humans:
(1) Buccal cavity
(a) Mouth:
- Food is ingested through mouth.
- Humans put food in the mouth through their hands and the digestion starts simultaneously.
- The mouth (buccal) cavity contains teeth, salivary glands and tongue.
(b) Teeth:
- Teeth cut the food into small pieces and chew and grind it.
(c) Salivary gland:
- The salivary gland secretes saliva ¡n our mouth. It is a watery liquid and so it wets (lubricates) the food in mouth. It is easy to swallow wet food.
- The salivary gland also secretes an enzyme called amylase. Amylase breaks the complex molecule called starch present in the food into sugar.
(d) Tongue:
- The tongue does the job of properly mixing the food with saliva.
- The food remains for a very short time in the mouth and hence only a part of food gets digested here.
![]()
(2) Oesophagus:
- The partly digested food comes down to oesophagus or say food-pipe.
- The main function of the oesophagus is to push the food down to stomach.
(3) Stomach:
- Stomach is a large organ which expands when the food enters it. Moreover, the stomach releases certain juices that help in digestion.
- The muscular walls of the stomach churn the food thoroughly with these digestive juices.
- During this process, the food is broken down into smaller pieces and is converted into a semi-solid paste.
- The wall of stomach contains three tubular glands which secrete gastric juice.
The gastric juice contains:
(A) Dilute hydrochloric acid, (B) An enzyme called pepsin and (C) Mucus
(a) Dilute hydrochloric acid:
- Since the stomach releases dilute hydrochloric acid, the digestive juices are acidic in nature.
- The presence of acid enables the enzyme pepsin to digest protein present in the food.
- Therefore, the function of hydrochloric acid is to create an acidic medium in the stomach.
- It also kills the bacteria that enter the stomach through food.
(b) Pepsin:
Pepsin is an enzyme that helps in digesting protein and converting food into smaller molecules.
(c) Mucus:
The mucus prevents the damage that the hydrocholic acki may cause to the inner lining of the stomach.
(4) Small Intestine:
- The partly digested food then moves from stomach to small intestine with the help of sphincter muscles. These muscles release the food in the small intestine in small parts.
- The small intestine is the longest part of the alimentary canal and hence an extensive coiled structure. Owing to this structure it can fit in a very compact space.
- The small intestine is the main site for complete digestion of carbohydrates, proteins and fats. It receives secretions from liver and pancreas for this purpose (whose functions are discussed below).
![]()
(a) Function of bile juice:
- Liver secretes bile juice.
- Bile is alkaline in nature and so it converts the acidic food coming from stomach into alkaline form so that enzymes of pancreas can act on it.
- In addition, the bile salts break the fats present in the food into small globules (droplets), which makes it easy for the enzymes to act and digest them.
(b) Function of pancreatic juice:
The pancreas secrete pancreatic juice, which contains enzymes such as amylase for digesting carbohydrates, trypsin for digesting protein and lipase for digesting fats.
(c) Function of intestinal juice:
- The glands of the wall of the small intestine secrete intestinal juice.
- The intestinal juice contains various enzymes, which complete the digestion of carbohydrates into glucose, proteins into amino acids and fats into fatty acids and glycerol.
Question 30.
Explain the process of absorption of food in human body.
Answer:
1. Absorption of food takes place in the small intestine after the food has been digested completely.
2. The inner wall of small intestine contains millions of small, finger-like projections called villi.
3. The presence of villi gives the inner walls of small intestine a very large surface area. This helps in the rapid absorption of digested food.
4. The villi possess numerous blood-vessels which take the absorbed food to each and every cell of the body.
5. The food absorbed in the cells is then used for obtaining energy, building new tissues and repairing old tissues.
Question 31.
Explain the process of assimilation of food and its egestion from the human body
Answer:
Assimilation :
- Blood receives food which is absorbed from the walls of small Intestine.
- Blood then carnes this food to all the parts of the body where it is assimilated by the cells.
- This assimilated food is used by all the cells for obtaining energy, growth arid repair of the body tissues.
Egestion:
- The unabsorbed food is sent to the large intestine.
- In the large intestine, most of the water of the undigested food is absorbed again by villi. This makes the undigested food almost solid.
- This solid form called faeces or stool is removed from the body through the anus.
- Sphincter muscles present in the anus regulate the removal of this waste material from the body.
Question 32.
What is the function of saliva?
Answer:
Salivary gland:
1. The salivary gland secretes saliva in our mouth. It is a watery liquid and so it wets (lubricates) the food in mouth. It is easy to swallow wet food.
2. The salivary gland also secretes an enzyme called amylase. Amylase breaks the complex molecule called starch present in the food into sugar.
Question 33.
Which gastric juices are released in our stomach? State their roles. OR Why does the stomach release hydrochloric acid?
Answer:
Stomach:
1. Stomach is a large organ which expands when the food enters it. Moreover, the stomach releases certain juices that help in digestion.
2. The muscular walls of the stomach churn the food thoroughly with these digestive juices.
3. During this process, the food is broken down into smaller pieces and is converted into a semi-solid paste.
4. The wall of stomach contains three tubular glands which secrete gastric juice.
Question 34.
Discuss the functions of Intestinal and pancreatic juices.
Answer:
Small intestine:
1. The partly digested food then moves from stomach to small intestine with the help of sphincter muscles. These muscles release the food in the small intestine in small parts.
2. The small intestine is the longest part of the alimentary canal and hence an extensive coiled structure. Owing to this structure it can fit in a very compact space.
3. The small intestine is the main site for complete digestion of carbohydrates, proteins and fats. It receives secretions from liver and pancreas for this purpose (whose functions are discussed below).
(a) Function of bile juice:
- Liver secretes bile juice.
- Bile is alkaline in nature and so it converts the acidic food coming from stomach into alkaline form so that enzymes of pancreas can act on it.
- In addition, the bile salts break the fats present in the food into small globules (droplets), which makes it easy for the enzymes to act and digest them.
![]()
(b) Function of pancreatic juice:
The pancreas secrete pancreatic juice, which contains enzymes such as amylase for digesting carbohydrates, trypsin for digesting protein and lipase for digesting fats.
(c) Function of intestinal juice:
- The glands of the wall of the small intestine secrete intestinal juice.
- The intestinal juice contains various enzymes, which complete the digestion of carbohydrates into glucose, proteins into amino acids and fats into fatty acids and glycerol.
Question 35.
What are villi (Singular — vlllus, Plural — vllli)?
Answer:
The Finger like projections or structures present on the inner surface of the small intestine are known as villi.
Function: Villi increases the surface area of intestine for better absorption of digested food.
Question 36.
How does food move In the alimentary canal?
Answer:
1. The food moves in the alimentary canal by a process called ‘peristalsis’.
2. The circular and longitudinal muscles of the alimentary canal undergo involuntary contraction and relaxation. This results in the wavy movement which is known as ‘peristalsis’.
3. Due to peristalsis, the food is pushed ahead and the passage behind the food gradually closes. This also prevents the backward flow of food.
Question 37.
Differentiate between gastric juice and bile.
Answer:
| Gastric juice | Bile |
| 1. Gastric juice is released from the three tabular glands of stomach. 2. It is not stored anywhere. 3. It is acidic in nature. 4. It consists of dilute hydrochloric acid, enzyme pepsin and mucus. 5. It makes the food acidic. | 1. Bile is secreted by liver. 2. It is stored in gall bladder. 3. It is alkaline in nature. 4. It contains bile acids and salts. 5. It makes the food alkaline. |
Question 38.
Explain how different organisms adopt different ways to release energy obtained from food material. OR Explain break-down of glucose by various pathways.
Answer:
1. The food material taken in during the process of nutrition is used in cells to provide energy for various life processes. The process of converting food into energy varies among organisms.
2. In any case, the first step is to break-down glucose which is a 6-carbon molecule, into two 3-carbon molecule called pyruvate. This process takes place in the cytoplasm.
![]()
Further conversion of pyruvate:
(1) In absence of oxygen (anaerobic respiration):
- Conversion of pyruvate in absence of oxygen is called anaerobic respiration.
- In the absence of oxygen, the conversion of pyruvate may take place in two ways. They are —
(a) The pyruvate may be converted into ethanol and carbon dioxide.
This process takes place in yeast during fermentation.
(b) When our muscles lack oxygen, the pyruvate gets converted into lactic acid which is also a 3-carbon molecule.
When we over-stress our muscles they build up lactic acid which then results in cramps.
(2) In presence of oxygen (aerobic respiration):
- The conversion of pyruvate in the presence of oxygen is called aerobic respiration.
- In aerobic respiration, pyruvate breaks down in the mitochondria. This process breaks up the three-carbon pyruvate molecule to give three molecules of carbon dioxide. Water is also formed in this process.
- Aerobic process releases much greater energy as compared to aerobic process.
- The energy released during cellular respiration is immediately used to synthesize a molecule called ATP which is used to fuel all other activities in the cell.
![]()
Question 39.
What is respiration? What are its types?
Answer:
Respiration:
The process of releasing energy from food substances such as glucose, amino acids and fats under the control of enzymes so as to carry Out various life processes by an organism is known as respiration. There are two types of respiration:
- Aerobic respiration
- Anaerobic respiration
![]()
Question 40.
Explain anaerobic respiration.
Answer:
Anaerobic respiration:
- The respiration which takes place in the absence of oxygen is known as anaerobic respiration.
- It is seen in microorganisms like bacteria, yeast, fungi, endoparasites and muscle cells.
(a) In yeast (plant):
During anaerobic respiration in yeast (plants), carbon dioxide and ethanol are formed as end products.
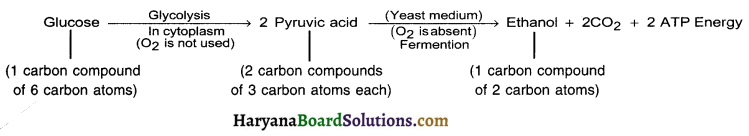
(b) In animal medium (In muscle cells):
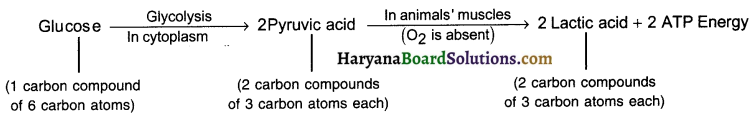
Question 41.
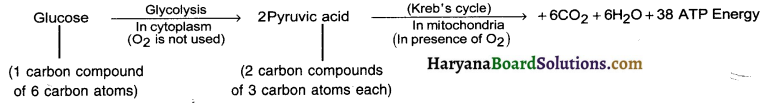
Explain aerobic respiration.
Answer:
Aerobic respiration :
- The respiration which takes place in the presence of oxygen is known as aerobic respiration.
- Most of the organisms show aerobic respiration.
- It takes place in the cells and so it ¡s also called cellular or internal respiration.
- During aerobic respiration, digested food (glucose), in the presence of oxygen, is completely broken down into carbon dioxide and water.
- The energy released during this process is stored in the form of ATP.
The overall equations is as under:
| Aerobic respiration | Anaerobic respiration |
| It takes place in presence of oxygen. | It takes paid in absence of oxygen. |
| End products are CO2 and water. | End products are ethanol or lactic acid. |
| It takes place in cytoplasm and mitochond ria. | It takes place only in cytoplasm. |
| Aerobic respiration produces a considerable amount of energy. | Anaerobic respiration produces quite less energy. |
Question 43.
Explain: Respiration In plants.
Answer:
Respiration in plants:
1. All plants need energy for their physiological processes. Like animals, plants also show gaseous exchange (or say respiration).
2. In plants, gaseous exchange takes place through stomata and the large inter-cellular spaces.
3. The gaseous exchange takes place through the process called diffusion. Diffusion mainly occurs through the surface of the plant leaves which is in direct contact with atmosphere.
4. The gases that are exchanged by diffusion are
- oxygen and
- carbon dioxide.
5. These gases may enter into plant cells or may move out of the plant cells into atmosphere. The direction of diffusion (i.e. movement of gases) depends upon
- environmental conditions and
- requirement of the plant.
6. In general, at night, when there is no photosynthesis, the plants eliminate maximum carbon dioxide (CO2) whereas during daytime plants release oxygen (O2).
![]()
Question 44.
Why do plant release oxygen during day time and carbon dioxide at night?
Answer:
Exchange of gases or say diffusion of gases is the process of respiration in the plants.
- Plants perform photosynthesis during day time. So, whatever carbon dioxide (CO2) is generate due to respiration gets used in the photosynthesis. Hence, there is no carbon dioxide remaining to release. As a result, plants only release oxygen.
- Photosynthesis does not occur at night and hence plants release carbon dioxide.
Question 45.
How do aquatic animals respire?
Answer:
1. For respiration, aquatic animals use oxygen dissolved in water.
2. The amount of oxygen dissolved in water is quite low as compared to amount of oxygen present in air. As a result, the aquatic animals have to put more effort to obtain oxygen required for respiration. So, the rate of breathing in aquatic organisms is quite fast as compared to terrestrial organisms.
3. Fishes take in water through their mouths and force it past the gills where the dissolved oxygen is taken up by the blood.
Question 46.
What are the basic characteristics of respiratory surface in terrestrial animals?
Answer:
Important characteristics of the respiratory surface of terrestrial animals:
- Terrestrial animals have large surface area of their bodies which remains in contact with oxygen rich atmosphere.
- This surface is very thin, fine and delicate. The thin walls facilitates diffusion of respiratory gases O2 and CO2 and also H2O in gaseous form.
- Usually, this surface lies within the body so that it can be properly protected. As a result there are passages that carry the oxygen to this surface from outside.
- There is also a mechanism for moving the air in and out of this area where oxygen is absorbed.
![]()
Question 47.
List out few organisms and their respiratory organs.
Answer:
Few organisms and their respiratory organs:
| Animals | Respiratory organs |
| Unicellular amoeba (Respire through process of diffusion) | Cell membrane |
| Earth worm | Skin |
| Insects | Trachea |
| Aquatic animals such as fish, prawn,crab and sepia | Gills |
| Frog, lizard, bird and humans | Lungs |
Question 48.
Explain the process of respiration in human beings along with the functions of the respiratory organs and a labelled diagram.
Answer:
Human respiratory system consists of nostril, nasal cavity, pharynx, Iaryngopharynx, trachea, bronchi, lungs, and diaphragm.
The process of respiration and functions of various respiratory organs of human beings are as follows:
1. Nose and nostrils:
- Human respiratory system starts with nose.
- Air is taken into the body from nostrils.
2. Nasal cavity:
- Nostril opens in the nasal cavity which is lined by fine nair and mucus.
- When air passes through the nasal cavity, the cavity traps the dust particles and microbes of the air and hence prevent them from entering the nose. Thus, filtered air moves further.
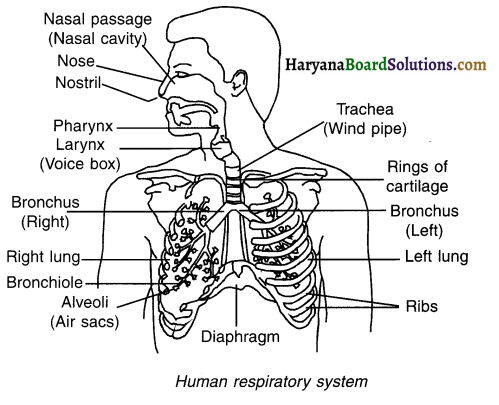
3. Throat:
- The air then passes through the throat and reaches the lungs.
- Cartilaginous rings are present in the throat. These rings ensure that the air passage remains open.
4. Lungs:
- Lungs are the chief respiratory organs.
- In the lungs, air passage divides into smaller and smaller tubes which finally end in balloon like structures called alveoli (or alveolar sacs).
- The walls of alveoli are thin and covered by blood capillaries. This provides a large surface for exchange of gases.
Question 49.
Explain the mechanism of breathing.
Answer:
1. The function of human respiratory system is to breathe in O2 and to breathe out CO2.
2. The alternate process of inspiration (inhalation) and expiration (exhalation) is known as breathing.
(A) Inspiration:
- During this process, we lift our ribs and flatten our diaphragm. As a result, the chest cavity expands.
- Due to this, the oxygen rich air from the atmosphere rushes into the lungs and fills the expanded alveoli sacs.
- The blood then takes this oxygen in the alveolar blood vessels and transports it to all the cells of the body.
(B) Expiration:
- The blood brings carbon dioxide from all the body cells and release into alveoli. The diaphragm relaxes i.e. moves up and the air containing carbon dioxide is pushed out of the lungs into the atmosphere through nostrils.
- This process is known as exhalation or expiration.
![]()
Question 50.
What is a respiratory pigment? Explain human respiratory pigment. Also state its importance.
Answer:
1. When the body surface of an animal is large, only diffusion pressure is not sufficient to transport oxygen to all the parts of the body. So, a specialized pigment called respiratory pigment does this work.
2. In human body, hemoglobin (Hb) which has a very high affinity for oxygen works as the respiratory pigment.
3. Hemoglobin is present in the red blood cells. It carries oxygen from the respiratory surface of the lungs to the body cells.
Question 51.
How is carbon dioxide transported towards lungs in human beings?
Answer:
Carbon dioxide is a respiratory gas to be exhaled out of the body. CO2 is more soluble in water than oxygen. So, majority of CO2 is transported in the dissolved form in blood plasma.
Question 52.
How are the lungs designed to increase the surface area for exchange of gases?
Answer:
Lungs:
- Lungs are the chief respiratory organs.
- In the lungs, air passage divides into smaller and smaller tubes which finally end in balloon like structures called alveoli (or alveolar sacs).
- The walls of alveoli are thin and covered by blood capillaries. This provides a large surface for exchange of gases.
Question 53.
Differentiate between breathing and respiration.
Answer:
| Breathing | Respiration |
| It is a mechanical process. | It is a physiological process. |
| Its cycle mainly consists of inhalation and exhalation. | It cycle includes glycolysis and Krebs cycle. |
| An organism may or may not have the mechanism of breathing. | The process of respiration occurs in each and every organism. |
| Breathing utilizes energy. | Respiration releases energy. |
Question 54.
State the role of blood in transportation. What are the basic requirements to run this transport system?
Answer:
Role of blood in transportation:
- Blood is a red coloured connective tissue in liquid form.
- Blood consists of fluid medium called plasma in which the cells remain in suspended form.
- Plasma does the work of transporting food, carbon dioxide, salts and nitrogenous wastes in dissolved form.
- The red blood cells transport oxygen.
Basic requirements to run this system:
- A pumping organ — To push blood around the body
- A network of tubes — To reach all the tissues
- A proper system —To ensure that this network can run properly and be repaired if damaged
![]()
Question 55.
Explain the structure of human heart along with a figure.
Answer:
1. Human heart is a muscular pumping organ to push blood around the body.
2. Its size is just as much as our fist.
Structure of human heart:
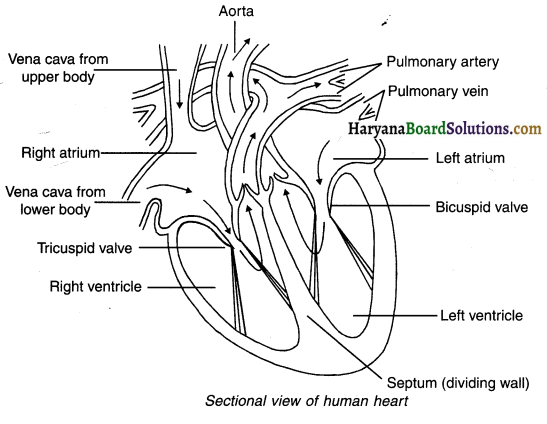
3. A human heart contains four chambers.
4. Both oxygen and carbon dioxide are transported by blood. So, in order to prevent the mixing of oxygen rich blood with blood containing carbon dioxide, there are four chambers in the heart.
The upper two chambers:
- These chambers are known as atria (singular : atrium).
- The left chamber is known as the left atrium while the right chamber is known as right atrium.
- The walls of atria are thin.
The lower two chambers:
- The lower chambers are known as ventricles.
- The left chamber is known as the left ventricle while the right chamber is known as the right ventricle.
- The walls of the ventricles are thick.
- All the four chambers are separated from each other by partitions called septa.
Valves:
- There is a bicuspid valve that allows the oxygenated blood to flow from left atrium to the left ventricle.
- Also, there is a tricuspid valve that allows deoxygenated blood to flow from right atrium to right ventricle.
- These valves prevent backward flow of blood from ventricles to the atria i.e. they are one-way valves.
![]()
Question 56.
Explain the flow of blood in human heart.
(Note : Although all the points of this answer are to be written as printed, but to understand the flow properly, read all the points marked as a’ together and then points marked as ‘b’ together.]
Answer:
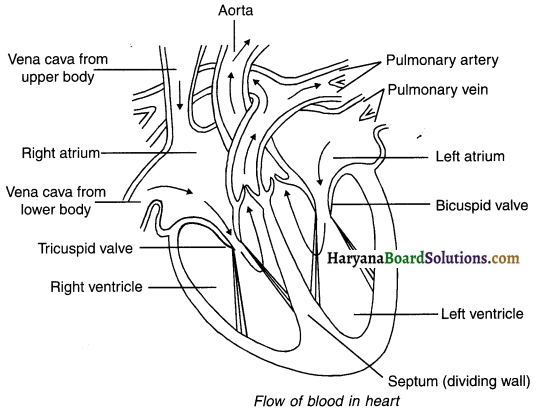
Initially, all the tour chambers of the heart are in relaxed state. This state of the heart is known as the diastolic stage.
- A-1. During this stage, the deoxygenated blood from various organs (except lungs) passes through superior and inferior venacava and reaches the right atrium.
- B-1. At the same time, the oxygenated blood from the lungs passes through pulmonary veins and reaches left atrium.
Now, both the atria contract and the following process takes place:
A-2. The tricuspid valve opens and deoxygenated blood from the right atrium is poured Into the right ventricle.
B-2. The bicuspid valve opens and oxygenated blood form the left atrium is poured into the left ventricle.
Now both the ventricles contract and the following process takes place:
A-3. Deoxygenated blood from the right ventricle passes from pulmonary valve and pulmonary arteries and reaches lungs.
- In lungs, CO2 is removed from blood and O2 from air is added into it.
B-3. Oxygenated blood from the left ventricle passes through aortic valve, aorta and pulmonary arteries and gets distributed to all the parts of the body (except lungs).
- Thus, the blood flows from right ventricle towards lungs and that from left ventricle towards all the parts of the body, expect lungs.
- Since the blood circulates twice through the heart, it is called double circulation.
- The separation of both types of blood in the heart allows a highly efficient oxygen supply system to the body.
Question 57.
With the help of a schematic diagram, show the transport and exchange of oxygen and carbon dioxide.
Answer:
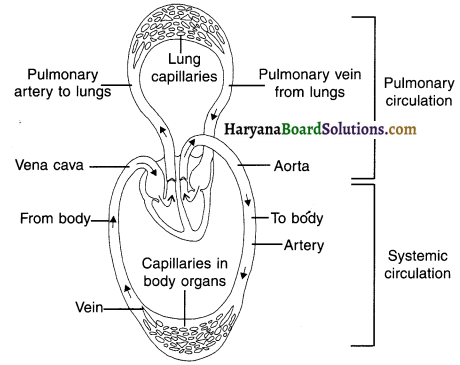
Schematic representation of transport and exchange of oxygen and carbon dioxide
![]()
Question 58.
Explain the evolution of heart starting from fishes to mammals. OR Explain the structure of hearts of lower animals (Note: For this question, write point (a) and (b) only).
Answer:
(a) 2-Chambered heart (Fishes):
- The heart of fishes have only two chambers.
- The blood is pumped to the grills. It is oxygenated there itself and passed directly to the rest of the body.
- Thus in fishes, the blood passes only once through the heart during one cycle of passage through the body.
(b) 3-Chambered heart (Amphibians and reptiles):
- In amphibians and many reptiles, there are three chambers in the heart. The oxygenated and deoxygenated blood gets mixed in the heart of such animals.
- Such organisms do not need high amount of energy like humans and so they can tolerate some Schematic representation of transport and exchange of oxygen and carbon dioxide
(c) 4-Chambered heart (Birds and mammals including humans):
- Birds and mammals have a four chambered heart.
- The separation of both types of blood i.e. oxygenated and deoxygenated in the heart allows a highly efficient oxygen apply system to the body.
- Such a system of separation is extremely useful for birds and mammals because they require very high energy for maintaining their body temperature.
Question 59.
Write a short note on blood vessels.
Answer:
Blood vessels:
Closed hollow tubes which transport blood from the heart to different organs and from different organs to the heart are called blood vessels. There are three types of blood vessels. They are:
(1) Arteries,
(2) Veins and
(3) Blood capillaries
(1) Arteries:
- Arteries carry blood from heart to all the organs of the body.
- For this, heart pumps blood into the arteries under high pressure.
- To sustain this pressure, walls of arteries are thick.
- After reaching the organs and tissues, arteries divide into many smaller vessels known as arterioles and finally into capillaries in order to make the blood reach to all the cells.
(2) Veins:
- Veins collect blood from different parts of the body and bring it back to the heart.
- Since blood is not under pressure while getting collected from the organs, the walls of veins are thin.
- Veins have valves to prevent backward flow of blood to ensure that the blood flows only in one direction.
(3) Capillaries:
- Capillaries are blood vessels having just a single-layered thick cell wall.
- Exchange of materials between blood and its surrounding talks place through these capillaries.
- These capillaries join to form venules and then veins.
Question 60.
What role do platelets play when we are Injured and bleeding? OR How does blood clot durIng an injury?
Answer:
1. If we get injured and our blood vessels rupture, the blood starts leaking form these vessels. This is called bleeding.
2. Due to leakage, the pressure of the blood will decrease. This will in turn decrease the efficiency of the pumping system i.e. the heart.
3. To avoid such a situation, blood contains platelet cells. The platelet cells keep on circulating around the body and plug the leaks in the vessels, if any. This clots the blood.
Question 61.
What is lymph? Write a short note on lymphatic system.
Answer:
1. Lymph is a colourless fluid which is also involved in transportation. It is also called tissue fluid. It consists of plasma and proteins.
2. Lymph does not contain red blood cells and so it is pale and colourless. Also, it contains less protein compared to blood.
Lymphatic system:
- The lymphatic system is a part of the immune system as well as circulatory system.
- Lymphatic system consists of lymph, lymph vessels, lymphatic capillaries and lymphatic nodes.
Flow of lymphatic system:
- Lymphatic flow begins in the areas around blood capillaries. In these areas, small amount of tissue fluid drains into lymphatic capillaries through its pores.
- The lymphatic capillaries then drain into larger lymph vessels that look like thin, transparent veins. Finally these veins open in large veins.
Functions of the lymphatic system:
- Lymph vessels carry digested and absorbed fats from intestine.
- It collects intercellular fluid through the medium of lymph vessels and returns ¡t to blood circulation.
- The system also protects against diseases.
![]()
Question 62.
Differentiate: Blood and lymph
Answer:
Blood | Lymph |
| Blood is a red coloured, living connective tissue. | Lymph is a colourless, liquid connective tissue |
| Blood plasma contains RBCs, WBC and Platelets. | Lymph plasma contains WBCs. |
| Blood contains more proteins. | Lymph contains less proteins |
| It clots quickly | It clots slowly |
| Blood flows throughout veins and carries oxygen to all the parts of the body. | Lymph works for removal of waste and other products that are released in the tissues. |
| It carries majority of digested food material. | It carries digested and absorbed fats from intestine towards the cells. |
Question 63.
Differentiate: Atria and ventricles
Answer:
Atria | Ventricles |
| The upper two chambers of a heart are called atria. | The lower two chambers of heart are called ventricles. |
| The walls of atria are thin. | The wall of ventricles are thick. |
| Blood moves out of atria with lesser pressure. | Pressure of blood from ventricles is higher. |
Question 64.
Differentiate: Arteries and veins
Answer:
Arteries | Veins |
| Arteries carry blood from the heart to different organs of the body. | Veins collect blood from different organs and send it to the heart. |
| Here, blood flows with high pressure. | Here, blood flows with a lower pressure. |
| Their walls are thick and elastic. | Their walls are thin and non-elastic. |
| Arteries do not have elastic valves. | Veins have a valve to prevent backward flow of blood. |
Question 65.
State the need of transportation system in plants.
Answer:
Need of transportation in plants:
1. Different types of substances that are absorbed or synthesized in one part of the body are transported to the various parts of the body. This is known as transportation.
2. Under photosynthesis, plants convert solar energy into chemical energy by utilizing carbondioxide and water.
3. Plants also need other substances which they take up separately by the means of roots. Roots absorb these substances from soil.
4. If the distance between roots and leaves is small, the raw material and energy that roots and leaves possess can easily reach all the parts of the body through simple process called diffusion.
5. But If the distance is long, process of diffusion becomes inefficient in transportation. In such a situation, a proper transport system is required. However, since plants do not move from one place to another, their energy requirement is lesser compared to animals.
Plants have two transport systems. They are:
(A) Xylem — For transporting water and minerals
(B) Phloem — For transporting food material produced by the plants
Question 66.
Explain transport of water In higher plants.
Answer:
Transport of water In higher plants:
1. Higher plants possess xylem for transporting water to all its parts.
2. Xylem contains tracheids and vessels.
3. Xylem tissues of all the organs of a plant are connected end to end with each other to form a network of conducting tubes.
Process of transport:
(a) Through diffusion:
- The cells of the roots are in direct contact with the soil and they take up ions from the soil.
- Due to this, a difference is created between the concentration of ions in the roots and that of ions in the soil.
- The ions present in the soil water are at higher concentration and so the water moves up from soil to the roots through osmosis.
- This water movement creates a water column under which water is steadily pushed upwards.
![]()
(b) Through conduction (transpiration):
- In higher plants transporting water till the highest point of the plant with this system is inefficient.
As a result, higher plants take help of a process called transpiration. - The transpiration or evaporation of water from the leaves create a suction which pulls up water from the xylem vessels.
- Thus the process of transpiration helps in the upward movement of water from roots to the leaves through the stem.
- Since the stomata are open during the daytime, the transpiration pull becomes a major driving force in the movement of water in the xylem.
Question 67.
Explain transpiration in brief.
Answer:
Transpiration:
1. The loss of water in the form of water vapour from the aerial parts of the plant is known as transpiration.
2. Plants absorb the water through the roots and transport it to all its parts.
3. This absorption is in fact driven by transpiration that occurs through the tiny pores on the leaves called stomata.
4. Transpiration creates a suction which pulls the water from the xylem cells of roots.
5. Thus, we can say transpiration plays an important role in making it possible for all the parts of the plant to receive water.
6. Transpiration also helps in regulating temperature.
Question 68.
What is translocation? Explain translocation of substances through the phloem tissue. OR How are food and other substances are transported In plants?
Answer:
Transportation of food and other substances:
1. The transportation of food and other substances i.e. the soluble products of photosynthesis is known as translocation.
2. Phloem is the chief tissue for conducting transportation.
3. Phloem transport the products of photosynthesis, some amino acids and other substances.
These products are transported from leaves to the other plant organs, while amino acids and the other products are transported towards the storage organs of roots, fruits, seeds and also to the growing parts of the plant.
4. Sieve tubes and companion cells are important components of the phloem. They can transport the food material in both directions i.e. upward and downward.
5. Sometimes translocation needs energy in the form of ATR in general, molecules like sucrose are transferred into phloem with the energy obtained from ATP. Here, the osmotic pressure in the cell increases, which pushes the food to the adjacent cell.
6. Interestingly in the spring season, the stored food in root or stem is transported to the buds to help them grow.
Question 69.
Differentiate: Xylem and phloem
Answer:
| Xylem | Phloem |
| Xylem transports water and mineral salts in the plants. | Phloem transports organic food materials in the plants. |
| Its transport route is from roots to leaves. | Its transport route is from leaves to various plant organs. |
| Here, transportation occurs in upward direction only. | Here, translocation occurs in both upwards as well as downward direction and also in lateral direction. In any and all directions. |
| Xylem contains tracheids and vessels. | Phioen contains sieve tubes and sieve cells. |
Question 70.
Differentiate: Transportation and translocation
Answer:
| Transportation | Translocation |
| The process of transporting water and other substances from one part of the plant to others is called transportation. | The process of transporting food and other substances from one part of the plant to others is called translocation. |
| In plants, xylem does the task of transportation. | Phloem does the task of translocation. |
| Xylem mainly transports water and some other substances. | Phloem transports food i.e. carbohydrate, amino-acids, plant hormones, etc. |
| Transpiration plays an important role in transportation. | Transpiration is not of much importance in translocation. |
| Transportation occurs from downward to upward direction only. | Translocation can occur in upward as well as downward and lateral direction. |
![]()
Question 71.
What is excretion? Discuss its need.
Answer:
Excretion:
- The harmful substances produced during several biochemical reactions are known as excretory substances.
- The biological process of removing these harmful nitrogen-based metabolic wastes in liquid form is known as excretion.
- Most excreted substances leave the body in the form of urine. Some of them are also lost in sweat and some in air that we breathe out.
Need for excretion:
- Body cells perform biochemical processes for sustaining life.
- During these processes, both useful as well as harmful toxic substances are produced.
- Accumulation of harmful substances in the body can harm the body and so they need to be removed from time to time.
- The process of removing excretory substances is simple in unicellular organisms.
- Unicellular organisms remove the excretory substances by simple diffusion from the body surface in the surrounding water.
- However, excretion is a complex process in multi-cellular organisms and so they possess special organs and even organ-system to perform excretion.
Question 72.
Explain excretory system of humans with a labeled diagram. OR Discuss the removal of urine from the body.
Answer:
Human excretory system is made up of the following organs:
(a) A pair of kidneys,
(b) A pair of ureter,
(c) A urinary bladder and
(d) A urethra
- Kidneys are located in abdomen on either side of abdomen. Kidney is bean shaped.
- Urine produced in the kidneys passes through the ureters into urinary bladder.
- The urinary bladder opens into urethra. Finally, urethra removes the urine outside the body.
Question 73.
Describe the structure of nephron, with labeled diagram.
Answer:
Nephron:
- Nephron is the main functional unit of kidney.
- Each human kidney possesses about 10 lakh nephrons.
Structure of nephron:
Bowman’s capsule:
- Each nephron has a double wailed cup-shaped bag at its upper end which is called Bowman’s capsule.
- The Bowman’s capsule contains a mass (bundle) of blood capillaries which is called glomerulus.
Tubule:
- The lower end of the Bowman’s capsule is called tubule.
- The part of tubule which is near the Bowman’s capsule is quite small and is called the neck.
- After the neck, the tubule becomes very narrow and coiled.
- This region of tubule consists of
(A) A proximal convoluted tubule,
(B) A Henle’s loop and
(C) A distal convoluted tubule. - The posterior end of nephron is known as collecting tube.
- Collecting tubule opens into renal pelvis.
- The renal pelvis opens into ureter.
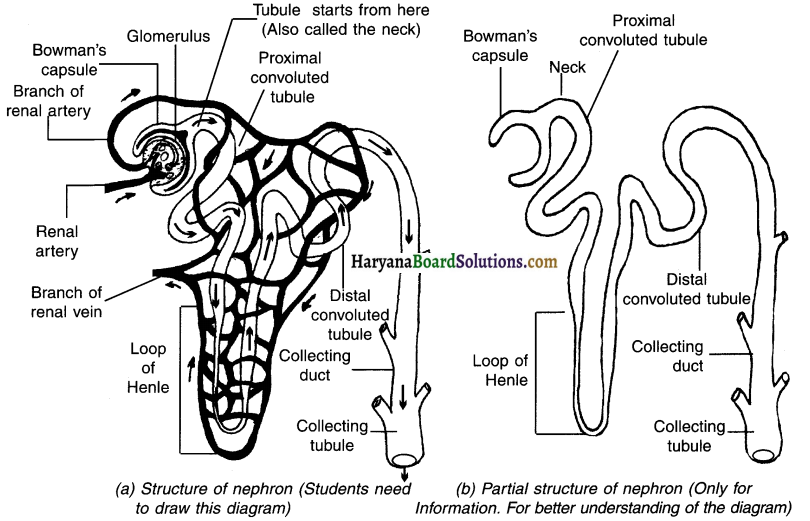
![]()
Question 74.
Explain urine formation in human kidneys.
Answer:
1. The purpose of making urine is to filter out waste products from the blood.
2. Urine contains nitrogenous wastes such as urea or uric acid which are removed from blood in the kidneys.
3. The renal arteries bring the waste material and the blood from the body to the kidney.
4. This blood is filtered out from the blood capillaries into the owman’s capsule.
5. This filtration takes place under heavy pressure and is known as ultrafiltration.
6. Each kidney has large numbers of filtration units called nephrons packed close together. The filtrate passes through the tubular parts of nephron.
7. During this, useful substances such as glucose, amino acid, salts and a major amount of water are reabsorbed by blood capillaries that surround the nephron.
8. The amount of water re-absorbed depends on how much excess water does the body contains and how much of dissolved waste is yet to be excreted.
9. The remaining fluid contains excretory substances called urine.
10. The urine passes from ureter to urinary bladder and gets stored there.
11. When the bladder is filled with urine it expands and creates a pressure which then creates an urge to pass the urine out of the body via, urethra.
Question 75.
How is urine produced?
Answer:
1. The purpose of making urine is to filter out waste products from the blood.
2. Urine contains nitrogenous waste such as urea or uric acid which are removed from blood in the kidneys.
3. It is then no surprise that the basic filtration unit in the kidneys, like in the lungs, is a cluster of very thin-walled blood capillaries.
4. Each capillary cluster in the kidney is associated with the cup-shaped end of a coiled tube called Bowman’s capsule that collects the filtrate
5. Each kidney has large numbers of these filtration units called nephrons packed close together.
6. Some substances in the initial filtrate, such as glucose, amino acids, salts and a major amount of water, are selectively re-absorbed as the urine flows along the tube.
7. The amount of water re-absorbed depends on how much excess water there is in the body and on how much of dissolved waste there is to be excreted.
8. The urine forming in each kidney eventually enters a long tube, the ureter, which connects the kidneys with the urinary bladder.
9. Urine is stored in the urinary bladder until the pressure of the expanded bladder leads to the urge to pass it out through the urethra.
10. The bladder is muscular, so it is under nervous control, as we have discussed elsewhere.
11. As a result, we can usually control the urge to urinate.
Question 76.
Explain excretion in plants.
Answer:
Excretion in plants:
1. For plants, oxygen is one of the end products of photosynthesis and can also be considered as a waste product.
2. Plants emit this oxygen into atmosphere through diffusion process.
3. Plants get rid of excess water through transpiration.
4. For other waste products, many of the plant tissues contain dead cells in themselves.
5. Some of the material is also lost by falling leaves.
6. In the plant cell, vacuoles are the excretory organelle.
7. Around the root system in the soil, plant excretes some of the waste products.
8. Peeling of the bark is also the example of plant excretion.
9. Resins and gums are excretions of plants.
![]()
Question 77.
Life on the earth depends on the sun. Give reason.
Answer:
1. All living beings need energy for their survival. They constantly need energy.
2. Food is the source of energy. This food directly or indirectly comes through the green plants.
3. All green plants trap solar energy or light energy and convert it in the form of food through the process of photosynthesis. Thus, the sun is the base of life on the earth.
Question 78.
Two green plants are kept separately in oxygen free containers, one In the dark and the other in continuous light. Which one will live longer? Why?
Answer:
1. The plant which is kept in dark will not be able to conduct photosynthesis. So, the container will soon be filled with the carbon dioxide released by the plant. Hence, the plant will die.
2. On the other hand, the plant kept in light would be able to carry out photosynthesis and thus convert the produced carbon dioxide into oxygen. Hence, this plant would live longer.
Question 79.
Leaves of a healthy potted plant were coated with Vaseline. Will this plant remain healthy for long? Give reasons for your answer.
Answer:
The plant covered with Vaseline will not remain healthy for long because Vaseline would make an impervious layer on the leaves. This will cause the following effects:
(a) Plant will not get oxygen for respiration.
(b) It will not get carbon dioxide for photosynthesis.
(c) Upward movement of water and minerals will be affected due to lack of transpiration.
Question 80.
Why mucus is secreted along with HCl in the stomach?
Answer:
Mucus makes a protective layer on the innermost layer of the stomach and protects it from the effect of HCl.
Question 81.
Why is small intestine in herbivores longer than in carnivores?
Answer:
1. Herbivores mainly eat green plants. So, cellulose found in green plants forms the largest part of an herbivore’s food.
2. Digesting cellulose takes quite a long time. Hence, herbivores have longer small intestine in which the food stays for a longer duration in order to get digested properly.
3. Carnivores do not eat plants and so their diet does not contain cellulose. So, the small intestine is small in them.
![]()
Question 82.
Why does absorption of digested food occur mainly in the small intestine?
Answer:
Maximum absorption of digested food occurs In small Intestine due to following reasons:
(a) Digestion is completed in small intestine.
(b) Inner lining of small intestine is provided with villi which increase the surface area to ensure better absorption.
(c) Wall of intestine is richly supplied with blood vessels which take the absorbed food to each and every cell of the body.
Question 83.
Hydrochloric acid Is an important constituent of gastric juice. Give reason.
Answer:
1. The stomach secretes gastric juices from its gastric glands for the chemical digestion of food.HCl is one of the constituents of gastric juice.
2. Hydrochloric acid destroys the bacteria and other microorganisms that enter along with the food and thus prevents the decay of food in stomach.
3. HCl creates acidic medium in the stomach so that the gastric enzyme can act properly.
Question 84.
Name the following —
(a) The process in plants that links light energy with chemical energy
(b) Organisms that can prepare their own food
(c) The cell organelle where photosynthesis occurs
(d) Cells that surround a stomatal pore
(e) Organisms that cannot prepare their own food
(f) An enzyme secreted from gastric glands in stomach that acts on proteins.
Answer:
(a) Photosynthesis
(b) Autotrophs
(c) Chloroplasts
(d) Guard cells
(e) Heterotrophs
(f) Pepsin
Question 85.
What is a residual volume of air in lung? What is its importance
Answer:
1. During the breathe-in cycle, when air is taken in and let out, some amount of air remains in the lungs. This is known as a residual volume of air in lungs.
2. The residual volume of air in lungs is important to provide sufficient time for oxygen to be absorbed and for carbon dioxide to be released. This way the exchange of respiratory gases can be carried out continuously.
![]()
Question 86.
Name the energy currency ¡n the living organisms. When and where is It produced?
Answer:
1. ATP (Adenosine Triphosphate) is the energy currency in the living organisms. It Is produced at the end of respiration in mitochondria.
2. The energy released during respiration is used to make an ATP molecule from ADP and inorganic phosphate.
![]()
Question 87.
The rate of breathing in aquatic organisms much faster than in terrestrial organisms. Give reason.
Answer:
1. Aquatic organisms like fishes obtain oxygen from water present in dissolved state through their gills.
2. The amount of dissolved oxygen is quite low in water as compared to the amount of oxygen in the air. So, the breathing-rate of aquatic organisms is much higher.
3. Terrestrial organisms use the oxygen present in the atmosphere for respiration. Oxygen is available in plenty in the atmosphere and so terrestrial organisms do not need to breathe very fast.
Question 88.
Give the main points of difference between respiration in plants and respiration in animals.
Answer:
Respiration in plants | Respiration in animals |
| All parts of plants such as roots, stems and leaves perform respiration Independently. | Specific organs or organ system. |
| Transport of gases occurs quite slow. | Transport of gases occurs quite fast. |
| Respiration in plants occurs at quite slower rate. | Respiration in animals occurs at a quicker rate. |
| Ethanol and carbon dioxide are the end products of respiration in plants. | Lactic acid is end the product of respiration |
![]()
Question 89.
Differentiate: Inhalation and Exhalation
Answer:
| Inhalation | Exhalation |
| To breathe-in air into lungs is called inhalation. | To breathe-out air from the lungs is called exhalation. |
| The size of chest cavity increases. | The size of chest cavity decreases. |
| The intercostal muscles and diaphragm contract. | The intercostal muscles and diaphragm relax. |
| The diaphragm comes downward. | The diaphragm goes upward. |
| The ribcage movement is upward and forward. | The ribcage movement is downward and inward. |
Question 90.
The walls of ventricles are thicker than atria. Give reason.
Answer:
1. The human heart is a constantly pumping organ.
2. The blood flows in very high force in ventricles as compared to atria. Hence, the walls of ventricles are thicker than atria.
Question 91.
What is the need of transportation in human beings? Why circulatory system is a need of human being?
Answer:
1. The circulatory system is very important to transport various substances required for life processes.
2. Circulatory system is important for transporting respiratory gases between lungs and body cells.
3. It is important to transport nutrients (digested) from alimentary canal to body cells.
4. It is important to transport excretory substances towards kidneys.
5. It is also important to transport some of the enzymes and various hormones.
Question 92.
Describe the functions of various blood cells.
Answer:
Functions of various blood cells:
(1) Red blood cells: They contain hemoglobin. Hence, they transport O2 from lungs to the body cells.
(2) White blood cells: They play an important role to provide immunity to the body. They protect the body from micro-organisms.
(3) Blood platelets: When any blood vessel gets cut, the platelets clot the blood and prevent bleeding.
![]()
Question 93.
What is the importance of the structure of septum in human heart?
Answer:
1. Human heart (and also birds) require high amount of energy. They also need to maintain their body temperature constantly. So, they need constant energy supply and hence constant supply of oxygen.
2. The septum separates the right side and the left side of the heart. As a result, the oxygenated blood and deoxygenated blood remain in separate chambers and do not mix.
3. Such a separation made by septum enables a very efficient supply of oxygen to the body cells.
Very Short Answer Type Question:
Question 1.
What is the Importance of life processes?
Answer:
To maintain the body functions of the living organism and to reproduce.
Question 2.
Name important life processes to maintain life.
Answer:
(i) Nutrition
(ii) Respiration
(iii) Transportation
(iv) Excretion
(v) Control and Co-ordination
(vi) Movement and
(vii) Reproduction.
Question 3.
What happens when the guard cells lose water?
Answer:
They shrink and cause the pore to close
Question 4.
In which from is carbohydrate stored?
Answer:
In plants, the carbohydrates are stored in starch form, while in animals, the carbohydrates are stored in glycogen form.
![]()
Question 5.
Which is the most common source to provide energy to the living organisms?
Answer:
Carbohydrates are the most common source for providing energy to the living organisms.
Question 6.
Give the summary of photosynthesis in terms of a reaction.
Answer:
6CO2 + 12H2O + Chlorophyll + Sunlight → C6H12O6 + 6O2+ 6H2O
Question 7.
Name the raw materials of photosynthesis.
Answer:
CO2. Water, Sunlight and Chlorophyll (a plant pigment)
Question 8.
Give any two examples of plant parasites.
Answer:
Cascuta and loxanthus
Question 9.
Give any two examples of animal parasites.
Answer:
Liverfiuke and plasmodium
Question 10.
What is the source of oxygen liberated during pbtosynthesis?
Answer:
Water
![]()
Question 11.
Give examples of plant with variegated leaves.
Answer:
The examples of plant with variegated leaves are money plant and crotons.
Question 12.
What is the site of photosynthesis?
Answer:
Chloroplast found in plant leaf
Question 13.
Define Translocation.
Answer:
Transfer of products of photosynthesis and some other materials through phloem is called as translocation.
Question 14.
A few drops of Iodine solution were added to rice water. The solution turned blue-black in colour. What does this indicate about rice water?
Answer:
The blue-black colour of rice water confirms the presence of starch
Question 15.
Which is the first enzyme to mix with food in the digestive tract?
Answer:
Amylase
Question 16.
What Is the importance of assimilated food in human body?
Answer:
(i) As a fuel to get energy,
(ii) As a material for growth and repair of the body.
![]()
Question 17.
Define Ingestion.
Answer:
The process by which the organisms take in the food is called ingestion.
Question 18.
Define Egestion.
Answer:
The process of removal of undigested food from the body is called egestion.
Question 19.
Define Peristalsis.
Answer:
The muscles of alimentary canal contract in a designed rhythm to push the food forward, This is called peristalsis.
Question 20.
In nasal cavity, which substance traps the dust particles and microbes present in the air?
Answer:
Mucus
Question 21.
What is the reason for muscular cramps after hard work?
Answer:
During hard work (or exercise) muscles undergo partial breakdown of glucose and forms lactic acid. This lactic acid is accumulated in the muscles and causes muscular cramps.
Question 22.
Give two examples of animals who breathe through their cell membranes?
Answer:
Amoeba. planarian and paramecium.
Question 23.
What is the average breathing rate in an adult man?
Answer:
The average breathing rate In an adult man is 15 to 18 breathe per minute.
Question 24.
What is the name of the area through which the exchange of respiratory gases occurs in a woody stem?
Answer:
Woody stems of plants/trees have lenticels for exchanging respiratory gases.
Question 25.
Which valve regulates the flow of blood from left atrium to left ventricle?
Answer:
Bicuspid value
![]()
Question 26.
Define Transpiration.
Answer:
The loss of water in the form of vapour through the aerial parts of the plants is called transpiration.
Question 27.
Give the location and function of a companion cell.
Answer:
The companion cells are located just next to sieve tube cells n a phloem tissue. They have nucleus and many other important organelles. They control the function of the sieve tube cell.
Question 28.
Define Pulmonary cIrculation.
Answer:
In human circulatory system, the blood circulatory system which transports blood between the lungs and the heart Is called as pulmonary circulation.
Question 29.
Define Systemic circulation.
Answer:
In human circulatory system, the transportation pathway of blood between various organs (except lungs) and heart is known as systemic circulation.
Question 30.
What is the pulse rate of a healthy adult person?
Answer:
The pulse rate of a healthy adult person is 72 per minute in resting position.
Question 31.
What are the mediums of circulation In human body?
Answer:
Blood and lymph are the mediums of circulation in human body.
![]()
Question 32.
Arrange these body parts starting from the lower side of the body.
I. Urter, II. Urethra, Ill. Kidney, IV. Renal vein
Answer:
III, I. Il, IV
Question 33.
Define Osmoregulatlon,
Answer:
The process of regulating waste contents and ion concentration in the body is called osmooegulation.
Question 34.
Name two chemical waste products of the human body.
Answer:
The two chemical waste products of the human body are urea and uric acid.
Question 35.
What is Urethra?
Answer:
Urethra is a tube through which urine is passed out from the body.
Question 36.
Define Dialysis.
Answer:
The artificial procedure used for cleaning the blood of a person by separating the waste substance (urea) from it is called dialysis.
Fill in the Blanks
1. Plants store food in the form of ……………..
Answer:
Starch
2. Each stoma consists of minute pore surrounded by a pair of …………..
Answer:
Guard cells
3. Guard cells are present in ……………. of leaves.
Answer:
Stomata
4. In amoeba, the process of obtaining food is called ………….
Answer:
Phagocytosts
![]()
5. The digested food found in food vacuoles of amoeba is absorbed directly into cytoplasm through ………….
Answer:
Diffusion
6. ………….. is the last step in the respiration process of human beings.
Answer:
Release of carbon
7. The process of food ingestion in amoeba is called …………
Answer:
Phagocytosis
8. ……………. is the largest gland of the body.
Answer:
Liver
9. Pepsin digests………….
Answer:
Proteins
10. In human beings ……….. is a respiratory pigment.
Answer
Hemoglobin
11. When air is blown from mouth into a test-tube containing lime water, the lime water turns milky due to the presence of …………
Answer:
Carbon dioxide
12. During respiration exchange of gases take place in ……………..
Answer:
Alveoli of lungs
13. Lack of oxygen in muscles often leads to cramps among cricketers. This results due to…………..
Answer:
Non-conversIon of glucose to pyruvate
14. The scientific name of voice box of humans is……………..
Answer:
Larynx
15. Blood is a …… liquid tissue.
Answer:
Connective
16. The ………. blood from various organs of the body is received by the right atrium.
Answer:
De-oxygenated
17. Capillaries join together to form ………….
Answer:
Veins
18. The normal rate of heart beat is ………………..
Answer:
72/mm
19. The normal blood pressure is …………..
Answer:
120/80 mmHg
![]()
20. The instrument to measure blood pressure is called ……………..
Answer:
Sphygmomanometer
21. The extracellular fluid which always flows front body tissues to the heart is called ………………..
Answer:
Lymph
22. At night ……………. is important for water transportation in plants.
Answer:
Root pressure
23. In nephrons, the structure of tubule after the neck is ………………..
Answer:
narrow and coiled
24. The chief nitrogenous waste products in the human beings are and ………………..
Answer:
Urea; uric acid
25. Oxygen liberated during photosynthesis comes from………………..
Answer:
water
26. Life on the earth depends on based molecules.
Answer:
Carbon
27. Plants excretory product(s) other than O2 and CO2 is/are …………………
Answer:
Resins and Gums
True Or False
1. A plant which is not growing visibly is dead. — False
2. Most of the food sources on earth are oxygen based. — False
3. Bacteria can produce their own food. — True
4. The opening and closing of the stomatal pore depends upon oxygen in guard cells. — False
5. The green dots on plant leaves are chloroplasts. — True
6. In amoeba, the undigested food moves to the surface of the cell. — True
7. Lipase is present in pancreatic juice. — True
8. During respiration in humans glucose is oxidized. — True
9. Pepsin is a protein digesting enzyme — True
10. Exit of food from stomach into small intestine as well as from anus to outside the body is regulated by sphincter muscles. — True
11. The internal (cellular) energy reserve in autotrophs is glycogen. — False
12. During deficiency of oxygen in tissues of human beings, pyruvic acid is converted into lactic acid in the chloroplast. — False
13. The direction of diffusion is not fixed. It depends upon the environmental conditions and the requirements of plants. — True
![]()
14. Among plants, humans and fishes, plants use oxygen at the fastest rate whereas fishes at the lowest rate. — False
15. Plants have specific respiratory pigments to in take oxygen from the atmosphere. — False
16. In pisces, the heart does not pump oxygenated blood to different parts of the body. — True
17. The blood leaving the tissues becomes richer in oxygen. — False
18. The left atrium receives oxygenated blood from the lungs through pulmonary vein. — True
19. There is independent pathway for transportation in plants. — False
20. Movement of material in xylem occurs through physical fores whereas movement of material in phloem occurs by utilizing energy. — True
21. Complex multi-cellular organisms remove the excretory substances by diffusion. — False
22. Bowman’s capsule brings the waste material along with the blood to the kidneys. — False
Match the Following :
Question 1.
| Column-I. | Column-II. |
| A. Unit of Respiration B. Unit of Excretion C. Unit of absorption of Digested food | P. Nephron Q. Villi R. Alveoli |
Answer: (A – R), (B – P), (C – Q)
Question 2.
| Column-I | Column-II |
| A. Transpiration B. Translocation C. Photosynthesis | P. Chloroplast Q. Stomata R. Phloem |
Answer: (A – Q), (B – R), (C – P)
![]()
Question 3.
| Column-I | Column-II |
| A. Carbohydrates B. Proteins C. Fats | P. Fatty acid + glycerol Q. Amino acid R. Glucose |
Answer: (A – R), (B – Q), (C – P)
Question 4.
| Column-I | Column-Il |
| A. Red blood cells B. White blood cells C. Platelets D. Lymph | R. Fat absorption from small intestine Q. Providing immunity R. Carrying O2 from lungs to body cells. S. blood clotting |
Answer: (A – R), (B – Q), (C – S), (D – P)
HBSE 10th Class Science Important Questions Chapter 6 Life Processes Read More »