Haryana State Board HBSE 10th Class Science Important Questions Chapter 3 Metals and Non-metals Important Questions and Answers.
Haryana Board 10th Class Science Important Questions Chapter 3 Metals and Non-metals
Question 1.
What are metals?
Answer:
1. Those elements which lose electrons to become positive ions are called metals. For example, magnesium (Mg) is a metal since it loses 2 electrons to become positive ion Mg2+.
2. Metals conduct heat and electricity.
3. They are hard, shiny, heavy and sonorous (i.e. make sound on banging).
4. Iron, copper, gold, aluminium, zinc, lead, etc. are all metals.
![]()
Question 2.
Discuss the physical properties of metals.
Answer:
Physical properties of metals :
1. Luster: Metals in their pure state have a shining surface. This property is called metallic luster. For e.g., gold, silver, etc.
2. Hardness: Most of the metals are hard. The hardness of various metals are different.
→ Interestingly metals like sodium and potassium are relatively soft and can be easily cut with a knife.
3. Malleability: Some metals can be hammered and turned into thin sheets. This property of metals is known as malleability. For e.g., gold, silver and aluminium.
4. Ductility: The ability of metals to be drawn into thin wires is called ductility. Metals are generally ductile.
5. Conductivity of heat: Metals are good conductors of heat.
6. Melting and boiling points: Metals have high melting and boiling points.
7. Electrical conductivity: Metals are good conductors of electricity. The power of conduction of heat and electricity is very high for copper, silver and gold metals.
8. Sonorous: Metals produce ringing sound on striking which means they are sonorous.
9. Alloys: Alloys can be prepared by adding one metal to the other. Brass, gold ornaments, stainless steel, etc. are the examples of alloys.
Question 3.
What is malleable and ductile? Explain.
Answer:
1. Some metals can be hammered and turned into thin sheets. This property of the metals is known as malleability.
2. This property is specially found in metals like gold, silver and aluminium. Hence, thin strips can be prepared from gold and silver and very thin paper-like foil can be prepared from aluminium.
3. Some metals are ductile i.e. we can draw thin wires from them. For e.g. gold and silver.
4. For example, about 2 kilometer long wire can be drawn from one gram gold. Wires can also be prepared from metals like copper and aluminium, by drawing.
Question 4.
Several metals conduct electricity even then mainly aluminium and copper are used for making electric wires. Why so?
Answer:
1. Almost all metals conduct electricity, but all metals are not available easily.
2. Metals like gold are very costly and metal like iron, rusts.
3. Aluminium and copper are two such metals which are easily available, cheap, and also do not rust. Hence, they are widely used in making electric wires.
![]()
Question 5.
What are non-metals?
Answer:
1. Elements that form negative ions by gaining electrons are called non-metals. For example, oxygen forms oxide ion O-2 by gaining electron and hence oxygen is a non-metal.
2. Carbon, sulphur, hydrogen are other examples of non-metals.
Question 6.
State physical properties of non-metals.
Answer:
Physical properties of non-metals:
- Non-metals are neither ductile nor malleable.
- Solid non-metals except iodine, are lusterless.
- Non-metals except diamond, are soft.
- Non-metals are bad conductors of heat and electricity. However, graphite is a good conductor of electricity.
Question 7.
“We cannot classify elements only on the basis of their physical properties.” Explain. OR State exceptions of metallic and non-metallic elements.
Answer:
(A) Exceptions found in metals:
- Although metals are solid but mercury exists as liquid at room temperature.
- Metals have high melting points but metals namely gallium and caesium have very low melting points.
- Alkali metals (lithium, sodium and potassium) are so soft that they can be cut with knife. Also, they have low densities and melting point.
(B) Exceptions found in non-metals:
- Non-metals exists as solids or gases at room temperature but bromine exists as a liquid.
- Non-metals do not possess lustre but iodine does.
- Non-metals have low melting point, but diamond, an allotrope of carbon has very high melting and boiling point.
- Generally, non-metals do not conduct electricity, but graphite, an allotrope of carbon is the only non-metal which conducts electricity.
- Owing to such exceptions we cannot classify metals or non-metals only on the basis of physical properties. Hence, they can be classified more clearly on the basis of their chemical properties.
![]()
Question 8.
What is allotrope? Give an example.
Answer:
Some elements possess the property of existing in two or more different forms in the same physical state. Such property is called allotropy. The different forms of the elements are called allotropes.
Example:
- Carbon, a non-metal exists in different forms and each of its form is called an allotrope.
- Diamond and graphite are two well-known allotropes of carbon.
Question 9.
Differentiate between metals and non-metals on the basis of their physical properties.
Answer:
|
Metals |
Non-metals |
| Metals are in solid form (except mercury). |
Non-metals are in solid, liquid or gaseous forms |
|
Metals are malleable and ductile. |
Non-metals are neither malleable nor ductile. |
| Metals are good conductors of heat and electricity. |
Non-metals are bad conductors of heat and electricity. |
|
Metals have luster. |
Non-metals are lusterless. |
| Most metals are heavy in weight. |
Most non-metals are light in weight. |
|
Generally, metals are hard. |
Generally, non-metals are soft. |
| Metals have higher melting or boiling points. |
Non-metals have lower melting or boiling points. |
|
Many metals produce ringing sound. |
Non-metals do not produce ringing sound. |
For information only:
Before studying the chemical properties of metals, let us take a basic idea of the reactivity series. The chemical properties of metals are determined by the way they react with oxygen, water, acid, etc. All metals do not react with a given element or a compound in the same manner. Some metals react vigorously, some react less vigorously and some do not react at all.
The question then is how do we determine if a metal will react with an element/compound? Or if it will react then in which manner? For this having a basic idea about the reactivity series of metals will make understanding the properties of metals quite easy.
![]()
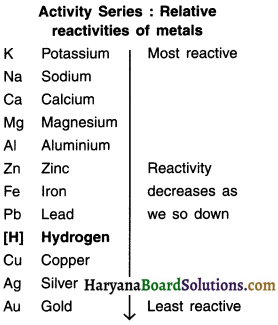
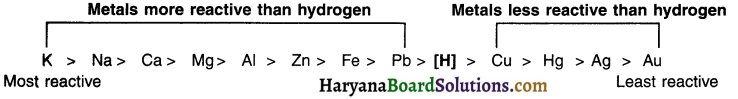
Activity (reactivity) series :
The arrangement of metals in decreasing order of their reactivities is called the activity or reactivity series of metals. The series is given in the adjacent column. Metals above hydrogen are more reactive whereas those below hydrogen are less reactive.
Potassium (K) and sodium (Na) are most reactive metals. Silver (Ag) and gold (Au) are least reactive metals. As we move down from K to Au, the reactivity decreases. This fact will play a very important role in determining the type of reaction that will take place between the metal and the element/compound.
Now let us understand the chemical properties of metals. Note that the points discussed in each property have been arranged in the decreasing order of reactivity i.e. from most reactive metal to the least reactive metal.)
Question 10.
How does metal reaòt with oxygen of air?
Answer:
Reaction of metals with oxygen (O2):
- Metals can easily give electrons to oxygen.
- Hence, metals combine with oxygen to form metal oxides.
Metal + Oxygen gas → Metal oxides
(1) Potassium (K) and sodium (Na) are so reactive that even if they are simply kept open in the room they react with oxygen (O2) of air. On reacting they start burning very vigorously and catch fire. Hence, they are stored immersed in kerosene.
(2) Metals such as magnesium, aluminium, zinc, etc. are covered with a thin layer of oxide. Hence, they do not react directly with air. So, we need to provide heat to for making them react with oxygen.
(3) Iron on heating in air reacts with the oxygen and forms oxides. Iron does not bum in air the way potassium and sodium does. However, iron fillings do burn vigorously.
(4) When copper s heated in air it combines with oxygen to form black coloured copper (II) oxide.

(5) Silver and gold are least reactive. They do not react with oxygen under any case.
Question 11.
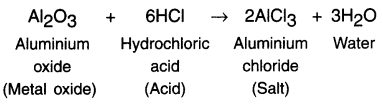
What are amphoterlc oxides? State examples
Answer:
Amphoteric oxides:
1. Some metal oxides react with both acids and bases to produce salt and water. Such metal oxides are called amphoteric oxides.
Example:
(i) Reaction with acid:
(ii) Reaction with base:
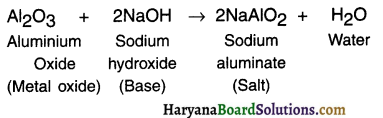
Question 12.
How do metal oxides react with water?
Answer:
Reaction of metal oxides with water:
Most metal oxides are insoluble in water. However, some dissolve in water and form alkalis.
Example: Sodium oxide and potassium oxide dissolve in water to produce alkalis.
(i) Na2O(s) + H2O(l) → 2NaOH(aq)
(ii) K2O(s) + H2O(l) → 2KOH(aq)
![]()
Question 13.
Discuss how metal reacts with water along with necessary chemical reactions.
Answer:
Reaction of metal with water (H2O):
1. Metals on reaction with water form metal oxides and produce hydrogen gas. Metal oxides that are soluble in water dissolve in it to further form metal metal hydroxide.
Metal + Water → Metal oxide + Hydrogen + Heat gas
Metal oxide + Water → Metal hydrxide (on dissolving)
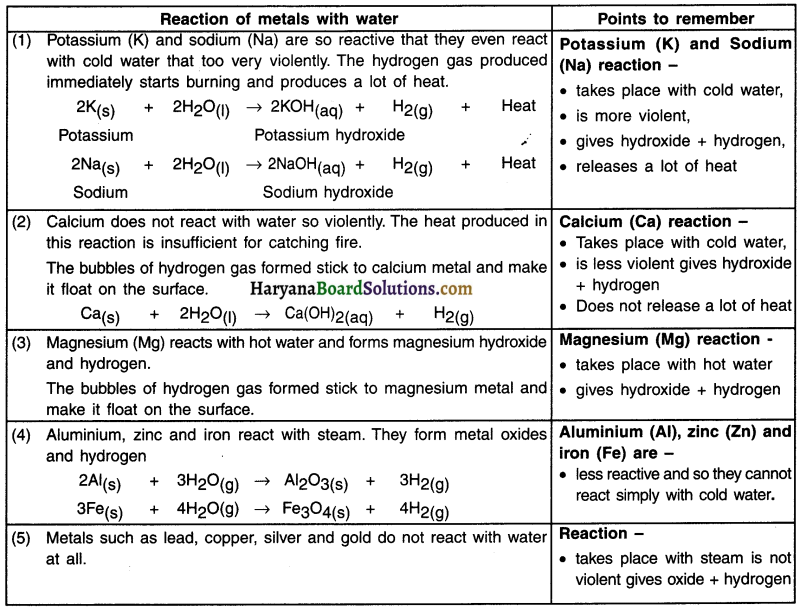
Question 14.
State and explain how metals react with dilute acid.
Answer:
Reaction of metals with dilute acid:
- All metals do not react with dilute acids.
- Those metals which react with dilute acids produce metal salt and hydrogen gas.
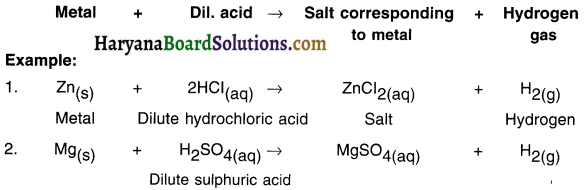
Hydrogen gas is liberated if the metal reacts with dilute hydrochloric acid (HCl), dilute sulphuric acid (H2SO4), etc. but, not with dilute nitric acid (HNO3). However, magnesium and manganese metals can react with very dilute nitric acid and produce hydrogen gas.
![]()
Question 15.
Why hydrogen gas is liberated when metals react with dilute hydrochloric acid and dilute sulphuric acid but not with nitric acid?
Answer:
1. When metals react with dilute nitric acid (HNO3), they do not produce hydrogen gas.
2. The reason behind this is that nitric acid is a strong oxidizing agent and so it oxidizes H2 produced during the reaction to H2O and its get reduced to any of the nitrogen oxides such as N2O, NO, NO2.
Question 16.
How will be the rate of liberation of hydrogen gas among various metals when they react with dilute acids?
Answer:
1. Metals above hydrogen in the reactivity series liberate hydrogen gas when they react with dilute mineral acid like hydrochloric acid, sulphuric acid. etc. (But not nitric acid).
2. Higher a metal in the reactivity series, the fastest will it liberate the hydrogen gas.
Example:
1. In the reactivity series, the elements magnesium (Mg), aluminium (Al), zinc (Zn), and iron (Fe) are placed above hydrogen in the sequence. Hence, the rate of liberation of hydrogen gas in the descending order will be Mg > Al > Zn > Fe.
Question 17.
Write equations for the reactions of magnesium, aluminium, zinc and iron with dilute hydrochloric acid.
Answer:
(i) Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
(ii) 2Al(s) + 6HCl(aq) → 2AlCl3((aq) + 3H2(g)
(iii) Zn(s)+ 2HCl(aq) → ZnCl2(aq) + H2(g)
(iv) Fe(s) + 2HCl(aq) → FeCl2(aq) + H2(g)
Question 18.
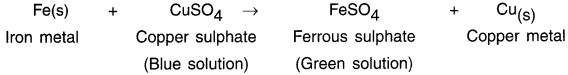
State and explain how metals react with solutions of other metal salts.
Answer:
Reaction of metals with solutions of other metal salts:
1. When metals react with solutions of salts of other metals, the more reactive metals displace less reactive metals from their salt solutions. This can be stated as follows:

Example: When a strip of iron metal is put into solution of copper sulphate CuSO4, the more reactive Fe metal displaces less reactive copper Cu metal.
![]()
Question 19.
State important chemical properties of metals without writing chemical equations.
Answer:
Chemical properties of metals:
1. Reaction of metals with oxygen (O2):
- Metals can easily give electrons to oxygen.
- Hence, metals combine with oxygen to form metal oxides.
Metal + Oxygen gas → Metal oxides
2. Reaction of metal with water (H2O):
- Metals on reaction with water form metal oxides and produce hydrogen gas. Metal oxides that are soluble in water dissolve in it to further form metal metal hydroxide.
Metal + Water → Metal oxide + Hydrogen + Heat gas
Metal oxide + Water → Metal hydroxide (on dissolving)
3. Reaction of metals with dilute acid:
- All metals do not react with dilute acids.
- Those metals which react with dilute acids produce metal salt and hydrogen gas.

4. Reaction of metals with solutions of other metal salts:
When metals react with solutions of salts of other metals, the more reactive metals displace less reactive metals from their salt solutions. This can be stated as follows:
Question 20.
What is displacement reaction? Out of copper and iron, which is more reactive? Why iron is placed above copper in the reactivity series?
Answer:
1. The reaction in which a more reactive element displaces (removes) less reactive element from its compound is called a displacement reaction.
2. Iron (Fe) is more reactive than copper (Cu) which means iron will react easily compared to copper. Hence, iron is place above copper in the reactivity series.
![]()
Question 21.
Write a detailed note on activity (reactivity) series of metals.
Answer:
Activity (reactivity) series:
The arrangement of metals in decreasing order of their reactivities is called the activity or reactivity series of metals.
Explanation:
- All metals do not react at same rate.
- Some metals are more reactive while others are less reactive.
- Those metals which lose electrons easily and form ions are called more reactive metals. For example, calcium (Ca). On the other hand, less reactive metals do not lose electrons easily. For example, gold (Au).
Question 22.
What Information can one gain from reactivity series of metals?
Answer:
One can gain the following Information from reactivity series of metals:
(1) A more reactive metal will displace a less reactive metal (i.e. a metal below it in the reactivity series) from its solution.
(2) Metals present at the top of the activity series are less electropositive and do not occur freely In nature. On the other hand, metals at the bottom of the series are more electropositive and generally occur freely in nature.
Question 23.
Give two differences between chemical properties of metals and non-metals.
Answer:
| Metals |
Non-metals |
| 1. Metals form basic oxides. 2. Metals can displace hydrogen atoms from their dilute acids. |
1. Non-metals form neutral oxides. 2. Since non-metals cannot react with dilute acids, they cannot replace hydrogen atoms from dilute acids. |
Question 24.
What is chemical bond? Give Its types along with one example of each.
Answer:
Chemical bond: The phenomenon through which atoms of a molecule of a compound attract each other and combine is known as a chemical bond.
There are two type of chemical bonds. They are :
(i) Ionic bond : The bond formed between a metal and a. non-metal is called an ionic bond. For example, sodium (Na) metal and chlorine (Cl) non-netal bond with each other through ionic bond and form NaCl (common salt).
(ii) Covalent bond : The bond formed between two non-metals is called a covalent bond. For example, two hydrogen (H) atoms join with each other through a covalent bond and form a hydrogen molecule (H2).
![]()
Question 25.
Explain how Na and Cl bond to form NaCl (Sodium chloride). OR Give an example to demonstrate how metals and non-metals react.
Answer:
1. Formation of NaCl:
Sodium metal (Na) and non-metal chlorine (Cl) combine with each other through ionic bond.
|
Element |
Type | Atomic No. |
Electronic Configuration |
|
(1) Sodium |
Metal | 11 | 281 |
| (2) Chlorine | Non-metal | 17 |
287 |
2. As can be seen in the table above, there is 1 electron in the outermost shell (orbit) of sodium (Na) metal.
3. The sodium metal will tend to lose 1 electron from its M shell. On doing so, ‘L’ will become the outermost shell with a stable octet.
4. The nucleus of this atom will still have 11 protons but the number of electrons after giving 1 electron will become 10. Thus, there is a net positive charge with sodium Na+.
5. Chlorine has 7 electrons ¡n its outermost. i.e. ‘M’ shell. It requires only 1 electron to complete its octet and become stable.
Reaction between sodium (Na) and chlorine (Cl):
1. If sodium and chlorine are reacted, sodium will lose its one electron which chlorine will take-up.
2. On gaining the electron, chlorine atom will get a negative charge, because its nucleus has 17 protons and 18 electrons. This will give us chloride anion Cl–.
Both, Na and Cl perform a give and take relation as follows:
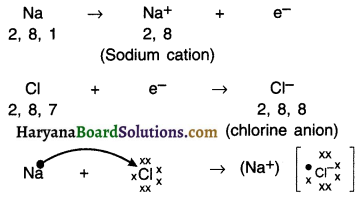
3. Sodium and chloride ions are oppositely charged and hence they attract each other and hold each other by strong electrostatic force of attraction.
4. Sodium chloride does not exist as an atom but as oppositely charged ions.
Question 26.
Explain the formation of ionic bond In magnesium chloride.
Answer:
1. Since magnesium’s atomic number is 12, its electronic configuration is (2, 8, 2).
2. Magnesium has 2 valence electrons in it and so it will tend to lose them, achieve octet configuration of nearby inert gas and form magnesium ion (Mg2+)

3. On the other hand, two chlorine (2,8,1) atoms will accept each of these electrons liberated by magnesium and form two Cl ions. Thus, chlorine will attain an octet configuration of (2, 8, 8).

4. Thus, the two electrons lost by magnesium atom are gained by two chlorine atoms (each one gets one electron) and forms magnesium chloride.
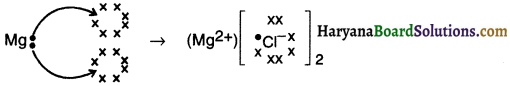
![]()
Question 27.
Give the properties of ionic compounds.
Answer:
Properties of Ionic compounds :
(1) Physical nature :
- Ionic compounds are obtained in solid form.
- The ionic compounds are hard due to strong force of attraction between positive and negative ions. Also, they are brittle and break into pieces on applying pressure.
(2) Melting point and boiling point :
In ionic compounds, positive and negative ions are joined strongly due to inter-ionic attraction. As a result, more energy is required to break such compounds and hence their melting and boiling points are high.
(3) Solubility :
Ionic substances (electrovalent compounds) are soluble in water but insoluble in organic solvents such as kerosene, petrol, etc.
(4) Conduction of electricity:
An ionic compound cannot conduct electricity in solid form. However, an ionic compound can conduct electricity when
- It is dissolved in water or
- When is in molten state.
5. The necessary condition for conducting electricity through a solution is that the charged particles should move in the solution.
![]()
Question 28.
Explain the conduction of electricity in ionic compounds.
Answer:
An ionic compound cannot conduct electricity in solid form. However, an ionic compound can conduct electricity when
- It is dissolved in water or
- When it is in molten state.
The necessary condition for conducting electricity through a solution is that the charged particles should move in the solution.
(a) in solids:
The ions cannot move and hence the electricity is not conducted. For example, in solid NaCl (common salt).
(b) ionic compound dissolved in water:
When ionic compounds dissolve in water, the solution so formed contain free-floating ions. When electricity is passed in this solution, the free-floating ions move towards the opposite electrodes. This confirms that ionic compound dissolved in water conducts electricity. For example, NaCl dissolved in water.
(c) Molten ionic compound:
To melt an ionic compound we need to supply heat. The heat overcomes the electrostatic force of attraction between the oppositely charged ions. As a result, the ions start moving freely and conduct electricity. For example, on heating NaCl (common salt). its molten form will conduct electricity.
Question 29.
Ionic compounds have high melting and boiling points. Give reason.
Answer:
Melting point and boiling point :
In ionic compounds, positive and negative ions are joined strongly due to inter-ionic attraction. As a result, more energy is required to break such compounds and hence their melting and boiling points are high.
Question 30.
Solid ionic compounds are bad conductor of electricity, but in aqueous solution they conduct electricity. Give reason.
Answer:
Molten ionic compound:
To melt an ionic compound we need to supply heat. The heat overcomes the electrostatic force of attraction between the oppositely charged ions. As a result, the ions start moving freely and conduct electricity. For example, on heating NaCl (common salt). its molten form will conduct electricity.
![]()
Question 31.
What is mineral ore and gangue?
Answer:
(i) Mineral:
- The elements or compounds that occur naturally in the earth’s crust are called minerals.
- Sea-water also contains salts of metals such as sodium chloride, magnesium chloride, etc.
(ii) Ores:
Those minerals from which the metals can be extracted, conveniently and profitably are called ores. (Note: Although all minerals contain metals, they are not termed as ‘ores’. The reason behind this is that it is not always practically possible to extract metals from these minerals. Hence, only those minerals in which metals is present in large quantity and from which metals can be extracted conveniently and reasonably are called ores.)
(iii) Gangue:
Impurities such as sand, mud, etc. present in the ore are called gangue.
Question 32.
All ores are minerals but all minerals are not ores. Give reason.
Answer:
(i) Mineral:
- The elements or compounds that occur naturally in the earth’s crust are called minerals.
- Sea-water also contains salts of metals such as sodium chloride, magnesium chloride, etc.
(ii) Ores:
Those minerals from which the metals can be extracted, conveniently and profitably are called ores. (Note: Although all minerals contain metals, they are not termed as ‘ores’. The reason behind this is that it is not always practically possible to extract metals from these minerals. Hence, only those minerals in which metals is present in large quantity and from which metals can be extracted conveniently and reasonably are called ores.)
Question 33.
How do metals occur On earth?
Answer:
(1) in free state (Least reactive metals):
- Only those metals which are very less reactive are found in the free state (or say native form) in nature. For example, silver (Ag), gold (Au) and platinum (Pt).
- Copper and silver are found in the form of oxides and sulphides also.
(2) in the form of compounds (Highly reactive metals):
Highly reactive metals such as potassium (K), sodium, Magnesium (Mg), Aluminium (Al), etc. are so reactive that they are never found in their free form in the nature. They are only found in the form of their compounds.
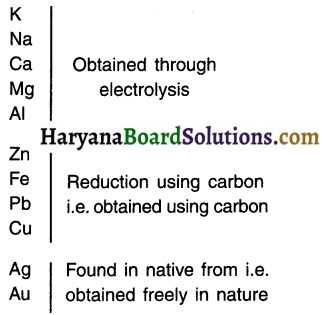
(3) In the form of oxides/sulphates/carbonates (Moderately reactive metals):
Moderately reactive metals such as zinc (Zn), Iron (Fe), Lead (Pbs, eth that lie at the ‘middle of the reactivity series are found in the earth in the form of oxides, sulphides and carbonates.
(Note: Textbook says that highly reactive minerals are found in the form of oxides, suiphides and carbonates. It is a mistake. This can be easily verified by studying section 3.4.4 Extracting Metals in the Middle of the Activity series of the textbook.)
In general —
- All the metals placed above copper (Cu) are found in nature only in the form of their compounds.
- Ores of many metals are found in the form of their oxides ie. the metal ore is oxide ore. This is because oxygen is a very reactive element and also it is found in abundance in the earth.
- Since, the reactivity of metals is different, the method to extract them from nature is also different.
- Extracting pure metals from their ores require several steps.
Question 34.
Explain In brief how are metals having different reactivities extracted from nature?
Answer:
(1) Least reactive metals:
Metals such as mercury (Hg) and copper (Cu) that lie in the lower section of the activity series are very unreactive.
They are extracted as follows:
(a) Since these metals are very less reactive they get extracted from their oxides simply by heating.
(b) Then, the metals are refined using electrolytic refining.
(2) Moderately reactive metals:
- Metals such as iron (Fe), zinc (Zn), lead (Pb), etc. lie at the middle of reactivity series and are moderately reactive.
- In nature, these metals occur in the form of suiphides and carbonates.
![]()
They are obtained in pure form as follows:
(a) The sulphide or carbonate ores are first heated (through roasting/calcination) to convert them into oxides.
(b) The metal oxides so formed are then reduced to their corresponding metals using reducing agents such as carbon (C).
(c) Finally, the metals are refined using electrolytic refining.
(3) Highly reactive metals:
Metals such as potassium (K), sodium (Na), calcium (Ca), magnesium (Mg), aluminium (Al), etc. are highly reactive and are placed at the top of the reactivity series.
They are obtained in pure form as follows:
(a) Since these metals are highly reactive, they are extracted using the method of electrolytic reduction or electrolysis. For example, sodium, magnesium and calcium are obtained by the electrolysis of their molten chlorides.
(b) Then, the metals are refined using electrolytic refining.
(Note: All metals extracted have one last common step which is ‘electrolytic refining’. This step removes all the impurities and finally gives us metal in its purest form. This method is discussed in text-book in section 3.4.6 Refining of Metals.)
Question 35.
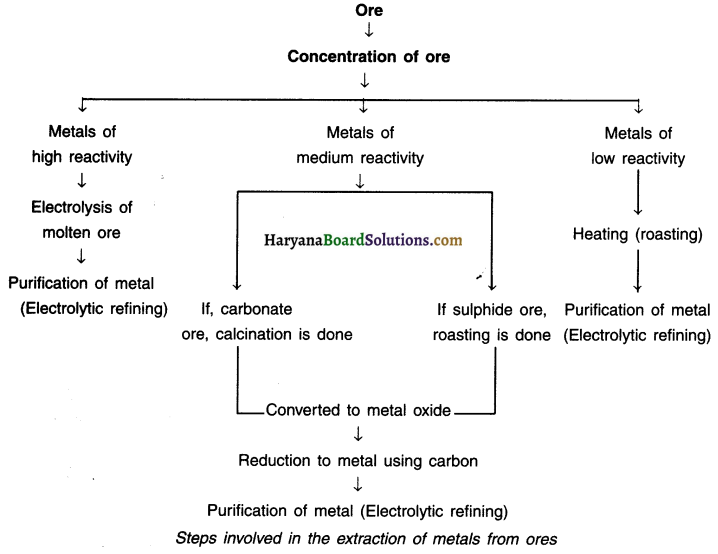
List out steps involved in extracting metal from its ore.
Answer:
The chart below shows steps for extracting metals from their ores.
Question 36.
How are metals low in activity series extracted from their ores? OR How are less reactive metals extracted from their ores?
Answer:
Metals that lie in the lower section of the activity series are very unreactive. As a result, they easily get extracted from their oxides simply by heating.
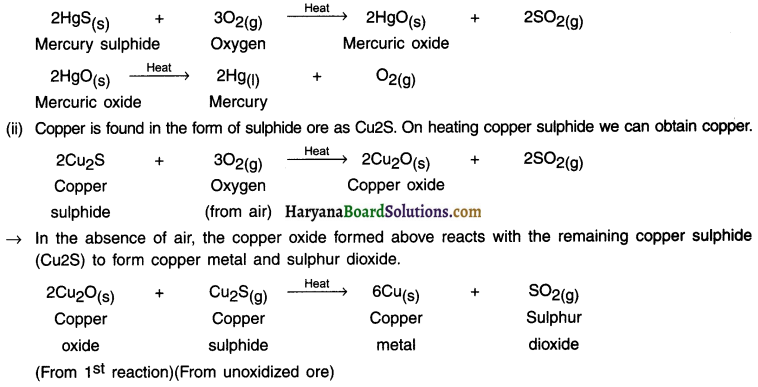
Example:
(i) Mercury sulphide (HgS) also known as cinnabar is an ore of mercury.
On heating it, it first gets converted into mercuric oxide (HgO). On heating (HgO), we can obtain mercury (Hg) in pure form.
Question 37.
How copper is extracted from Its ore?
Answer:
1. Copper is a very less reactive metal. Hence, it is obtained from its ore simply by heating.
2. The chart below shows steps for extracting metals from their ores.

Question 38.
How are metals lying in the middle of activity series extracted?
Answer:
1. Metals such as iron (Fe), zinc (Zn), lead (Pb), etc. lie at the middle of reactivity series and are moderately reactive.
2. In nature, these metals occur in the form of sulphide and carbonates.
3. It is easier to obtain metals from their oxides rather than sulphides and carbonates. Hence, the moderately reactive metals are first converted into oxides. This is done as follows:
![]()
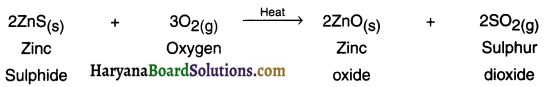
(a) Roasting:
1. This process is used for suiphide ore. Roasting is the process in which the sulphide ore is heated strongly in the presence of excess air to convert it into metal oxide.
Example:
(b) Calcination:
1. This process is used for carbonate ore.
2. Calcination is the process in which the carbonate ore is heated strongly in the limited air to convert it into metal oxide.
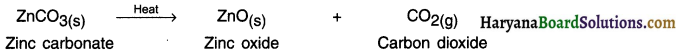
3. The metal oxides obtained by calcination or roasting are then reduced (converted) into free metal by using reducing agents such as carbon, aluminium, sodium or calcium. The reducing agent to be used again depends on the chemical reactivity of the metal to be extracted.
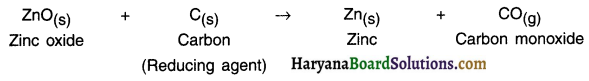
(i) Reduction of metal oxide using carbon:
The oxides of comparatively less reactive metals like zinc, iron, lead, etc. are reduced using carbon as the reducing agent.
(ii) Reduction using aluminium: If the metal oxide is of more reactive metal, then aluminium can be used as a reducing agent to extract metal from its metal oxide. The reason for using aluminium is that aluminium is a more reactive metal and hence it can displace a comparatively less reactive metal from its metal oxide to give free metal.
(iii) Manganese and iron (Fe) metal is extracted from its oxide using aluminium.

(iv) Note that this reaction is a displacement reaction as well as reduction and oxidation reaction.
(v) Such displacement reactions are highly exothermic.
![]()
Question 39.
What s thermite reaction? Give one example.
Answer:
Thermite reaction:
1. The process or reaction in which a metal is reduced from its oxide using aluminium powder as a reducing agent is called thermite reaction (or thermite process).
2. Thermite reactions are highly exothermic and produce so much heat that the metals are obtained in molten state.
Example:
Iron oxide is reacted with aluminium to obtain iron metal in molten state. The molten iron is then used to weld the railway tracks and machine parts.
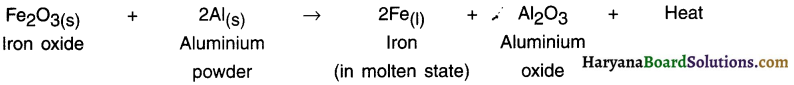
Question 40.
What is roasting?
Answer:
Roasting:
1. This process is used for sulphide ore. Roasting is the process in which the sulphide ore is heated strongly in the presence of excess air to convert it into metal oxide.
Example:

Question 41.
What is calcination?
Answer:
Calcination:
1. This process is used for carbonate ore.
2. Calcination is the process in which the carbonate ore is heated strongly in the limited air to convert it into metal oxide.
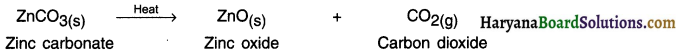
3. The metal oxides obtained by calcination or roasting are then reduced (converted) into free metal by using reducing agents such as carbon, aluminium, sodium or calcium. The reducing agent to be used again depends on the chemical reactivity of the metal to be extracted.
Question 42.
Explain method to obtain metal using carbon.
Answer:
Reduction of metal oxide using carbon:
The oxides of comparatively less reactive metals like zinc, iron, lead, etc. are reduced using carbon as the reducing agent.

Question 43.
Explain method to obtain metal using aluminium.
Answer:
Reduction using aluminium: If the metal oxide is of more reactive metal, then aluminium can be used as a reducing agent to extract metal from its metal oxide. The reason for using aluminium is that aluminium is a more reactive metal and hence it can displace a comparatively less reactive metal from its metal oxide to give free metal.
![]()
Question 44.
How are metals present in the top of activity series extracted?
Answer:
1. Metals such as potassium (K), sodium (Na), calcium (Ca), magnesium (Mg), aluminium (Al), etc. are highly reactive and are placed at the top of the reactivity series.
2. The oxides of these metals have more affinity i.e. attraction towards oxygen rather than carbon.
3. Hence, a method called ‘electrolytic reduction or electrolysis’ is used to extract highly reactive metals from their molten chlorides or oxides.
4. Sodium, magnesium and calcium are obtained by the electrolysis of their molten chlorides. Aluminium is obtained by the electrolytic reduction of aluminium oxide.
5. The metal obtained will be at cathode (negative electrode) because metals are positively charged and get attracted to negatively charged electrode (the cathode).
Following reactions take place during electrolysis of sodium chloride to obtain sodium metal:
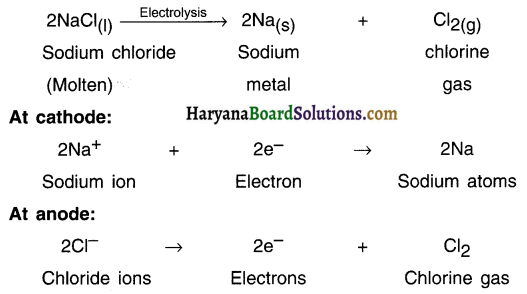
Question 45.
How is sodium metal extracted? OR State the reactions taking place during extraction of sodium.
Answer:
1. Sodium is a very reactive metal. Hence, it is extracted with the help of ‘electrolytic reduction (electrolysis)’ method.
2. Metals such as potassium (K), sodium (Na), calcium (Ca), magnesium (Mg), aluminium (Al), etc. are highly reactive and are placed at the top of the reactivity series.
3. The oxides of these metals have more affinity i.e. attraction towards oxygen rather than carbon.
4. Hence, a method called ‘electrolytic reduction or electrolysis is used to extract highly reactive metals from their molten chlorides or oxides.
5. Sodium, magnesium and calcium are obtained by the electrolysis of their molten chlorides. Aluminium is obtained by the electrolytic reduction of aluminium oxide.
6. The metal obtained will be at cathode (negative electrode) because metals are positively charged and get attracted to negatively charged electrode (the cathode).
![]()
Question 46.
Explain the procedure to perform electrolysis (electrolytic refining) of metal.
Answer:
Electrolytic refining:
Refining metals using the method of electrolysis is called electrolytic refining.
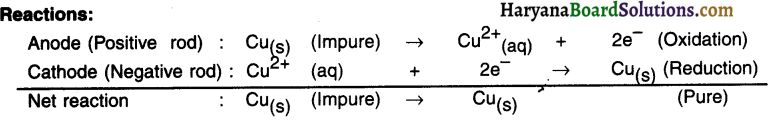
Electrolysis:
1. A thick rod of impure metal is taken as anode. It is connected to the +ve terminal of the battery.
2. A thin rod of pure metal is taken as cathode. It is connected to the – ve terminal of the battery.
3. Aqueous acidified solution of salt of metal to be refined (i.e. impure metal) is taken as an electrolyte and filled in the electrolytic vessel.
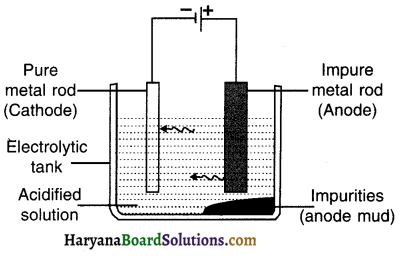
Procedure:
- After setting up the apparatus as shown in the figure, electricity is supplied.
- On passing the electric current through electrolyte, pure metal from anode dissolves in the solution i.e. the electrolyte. An equivalent amount of pure metal from the solution will get deposited at cathode.
The impurities that move out of the anode rod get divided Into following two parts :
(A) Soluble impurities go into the aqueous solution and
(B) Insoluble impurities get collected below the anode. It is then called anode mud or anodic mud.
Application :
To refine metals such as copper, zinc, gold and silver.
Question 47.
Explain the electrolysis of copper. OR explain procedure to obtain pure copper from its ore using electrolytic reduction.
Answer:
Electrolysis of Copper
- A thick rod of copper (Impure metal) to be purified is taken as anode and it is connected with the positive terminal of the battery.
- AcomparatlvelythlnrodofpurecOpperlstaken as cathode and it is connected with the negative
terminal of the battery - Aqueous solution of copper sulphate (salt of copper) is taken as electrolyte. Also, a dilute sulphuric acid Is added to It
- The copper sulphate solution then becomes acidified copper sulphate solution.
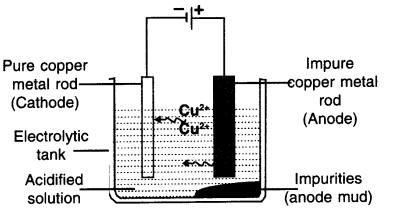
Procedure:
- After setting up the apparatus as shown in the figure, electricity is supplied.
- The positively charged copper ions i.e. Cu+2 will get attracted towards negatively charged electrodes i.e. cathode.
- This way pure metal cathode will become thick.
- The impure copper goes into the solution in the form of ions.
![]()
The Impurities that move out of anode of copper gets divided Into following two parts:
(A) Soluble impurities go into the aqueous solution
(B) Insoluble impurities get collected below the anode. It is then called anode mud or anodic mud.
The copper deposited at cathode is almost hundred percent pure.
Question 48.
What is corrosion ? State Its advantages and disadvantages.
Answer:
Corrosion:
- The erosion reaction that takes place between a metal and water or moisture when they come in contact with each other called metallic erosion or corrosion.
- We can see the effect of air and water i.e. corrosion on surface of many metals.
Examples:
1. If objects made out of iron remain exposed to moist air for a longer period, their surface becomes brown and flaky. This is nothing but corrosion.
2. Silver articles or ornaments become black after sometime due to exposure to air.
3. A green coloured layer of copper carbonate forms on the surface of copper objects when they remain exposed in air for a long time.
4. Inert metal such as gold, never get corroded.
Disadvantages of corrosion:
- Every year, corrosion causes loss of crores of rupees in the world.
- Corrosion also reduces life of objects.
Question 49.
Enlist ways to prevent corrosion of iron. Explain the methods of galvanizing and making alloys for preventing corrosion.
Answer:
Corrosion (rusting) of iron can be prevented by painting, oiling, greasing, galvanizing, anodizing or making alloys.
(a) Galvanizing:
- Rusting of iron can be prevented by applying a coat of very fine layer of zinc metal on it.
- The process of applying zinc is called galvanizing and the iron on which it is applied is then called galvanized iron.
- For example, iron sheets used in the roofs of house are galvanized iron sheets.
(b) Making alloys:
- Another effective way to prevent corrosion is to change the properties of metals and non—metals. This can be done by mixing different metals and non-metals.
- For example, stainless steel is an alloy which is formed by adding chromium and nickel to iron.
This alloy does not get affected by air, water or alkali and it does not even get corroded. - Hence, utensils used in kitchen, instruments used in surgery, big vessels used in industries, etc. are prepared from stainless steel.
![]()
Question 50.
What is an alloy? State its preparation and some examples.
Answer:
Alloys:
- An alloy is a homogeneous mixture of two or more metals or metal and non—metal.
- There are several advantages of using alloys over metals.
Preparation:
- For making alloy, first of all the chief metal e. the metal whose alloy is to be made is melted.
- Then the substance which is to be mixed is added in definite proportion and the mixture is melted again.
- Then this molten mixture is allowed to cool to obtain alloy.
Examples:
- On adding a very small amount of carbon (about 0.05%) to iron, it becomes quite hard and strong.
- Stainless steel is obtained on adding nickel and chromium to iron. This steel is strong and does not corrode.
- The alloy prepared by adding zinc metal to copper is known as brass.
- Amalgam is an alloy made by mixing mercury with any metal.
Question 51.
State advantages of alloys with the help of examples.
Answer:
Advantages of alloys:
1. On adding a very small amount of carbon (about 0.05%) to iron, it becomes quite hard and strong.
2. Stainless steel is obtained on adding nickel and chromium to iron. This steel is strong and does not corrode.
3. The alloy prepared by adding zinc metal to copper is known as brass.
4. Brass is used in making cooking vessels, parts of machinery, musical instruments, etc.
5. An alloy which contains mercury is called amalgam.
6. The electrical conductivity of an alloy is less than the pure metal. For example, impure copper has a lesser conductivity compared to pure copper.
7. Melting point of an alloy is less than those of component elements. For example, melting point of the alloy prepared from lead and tin is less and so it is used in soldering electric wires
Question 52.
State important differences between diamond and graphite.
Answer:
|
Diamonds |
Graphite |
| 1. Diamonds are lustrous and transparent 2. Diamond is very rare and expensive 3. Diamond is extremely hard 4. It conducts heat |
1. Graphite is opaque and non-lustrous |
Question 53.
A non-metal X exists In two different forms Y and Z. Y Is the hardest natural substance, whereas Z is a good conductor of electricity. Identify X, Y and Z.
Answer:
1. The non-metal X is carbon (C). Carbon exists in two different forms called the allotropes of carbon. These allotropes are diamond and graphite.
2. The hardest natural substance is diamond. Hence, Y is diamond. Graphite is good conductor of electricity. Hence, Z is graphite.
![]()
Question 54.
Individually, hydrogen gas lacks the capability of combustion. Give reason.
Answer:
1. If you put an ignited match stick into glass jar, it extinguishes. But, if you allow oxygen to mix with hydrogen gas and then try to ignite t, it will ignite with a blue flame.
2. This suggests that hydrogen becomes combustible in presence of oxygen where as individually it is non-combustible.
Question 55.
A metal A, which is used in thermite process, when heated with oxygen gives an oxide B, which is amphoteric in nature. Identify A and B. Write down the reactions of oxide B with HCl and NaOH.
Answer:
1. Metal A is aluminium (Al) which is used in thermite process.
2. Aluminium reacts with oxygen to form aluminium oxide Al2O3 i.e. B which is amphoteric in nature.
4Al + 3O2 → 2Al2O3(s)
Reactions of oxide B i.e. Al2O3 with HCl and NaOH:
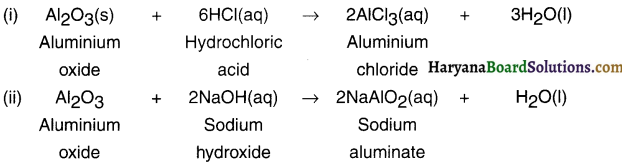
Question 56.
lqbal treated a lustrous, divalent element M with sodium hydroxide. He observed the formation of bubbles in reaction mixture. He made the same observations when this element was treated with hydrochloric acid. Suggest how he can identify the produced gas. Write chemical equations for both the reactions.
Answer:
The divalent element M that lqbal used is zinc Zn.
(a) Reaction of zinc with sodium hydroxide:
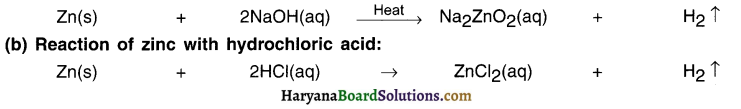
The gas produced gas can be identified by bringing splinter near the gas. The gas will start burning with a pop sound. This suggests that it is hydrogen gas.
Question 57.
A solution of CuSO4 was kept in an iron pot. After few days the iron pot was found to have a number of holes in it. Explain the reason in terms of reactivity. Write the equation of the reaction involved.
Answer:
The solution of CuSO4 means solution of copper sulphate. Iron is a more reactive metal than copper. Hence, it displaces copper from copper sulphate solution.
Reaction:
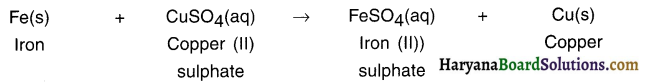
Since, iron takes part in this reaction, it produces holes at places where iron metal has reacted to form iron (II) sulphate.
Question 58.
The principle ‘Less active metals can be displaced from the solution of its salt by the more active metal’ is used ¡n developing the activity series of metals. Explain.
Answer:
1. For preparing the activity series of metals, the metals need to be arranged in decreasing order of their reactivities.
2. For this, it is necessary to determine which metal is more reactive or less reactive than others.
3. Certain reactions are carried out to determine the reactivity level.
4. The reactions can be done either with oxygen, water or even acid. But, all metals cannot undergo reactions using these methods.
5. To overcome the above problem, displacement reaction is carried out on the metals and then their reactivities are
compared.
6. Displacement reaction works on the principle ‘Less active metals can be displaced from the solution of its salt by more active metal’. As a result, this principle is used to determine the activity series.
![]()
Question 59.
‘Hydrogen is not a metal even then it is placed in the reactivitý series of metals’. Why?
Answer:
1. The reactivity of metals depends on the ease with which they can lose electrons.
2. Hydrogen is not a metal but still it can lose one electron quite easily compared to many other metals and form positive ions, Hence, hydrogen is placed in the reactivity series of metals.
Question 60.
A non-metal A is an important constituent of our food and forms two oxides B and C. Oxide B is toxic whereas C causes global warming.
(a) Identify A, B and C (b) To which group of Periodic Table does A belong?
Answer:
(a) Non-metal A is carbon. Its two oxides are carbon monoxide (CO) and carbon dioxide. Oxide B is carbon monoxide (CO) as it is toxic while C is carbon dioxide (CO2), because it is responsible for global warming.
(b) Atomic weight of non-metal A is 6. So, its electronic configuration is 2, 4. It is present in 14th group (10 + valence electrons), i.e. group IV of the Periodic Table.
Question 61.
‘Bonding alters the properties of elements.’ Explain this statement by giving examples.
Answer:
1. Sodium metal is highly reactive. It easily reacts with oxygen (O2) of the air and forms sodium oxide (Na2O). Na2O further reacts with water and gives sodium hydroxide (NaOH).
2. Sodium metal is highly active because its configuration is (2, 8, 1) and so it has a tendency to lose one electron from its valence orbit and thus form sodium ion (Na+).
3. Na+ attracts chloride ion (Cl–) which has a negative charge. Thus, Na+ and CF– combine and form NaCl.
4. Even though individually Na+ and Cl– are reactive and non-edible, when they combine by bonding, they lose their original individual properties and form NaCl i.e. common salt which can be consumed without any harm.
5. Thus, bonding alters the properties of elements.
Question 62.
Why should the metal suiphides and carbonates be converted to metal oxides in the process of extraction of metal from them?
Answer:
1. In metallurgy, it is easier to obtain metals from their oxides (by reduction) rather than from carbonate or suiphide ores.
2. As a result, before reduction the ore must be converted into metal oxide and then be reduced.
Question 63.
A metal that exists as a liquid at room temperature is obtained by heating its sulphide in the presence of air. Identify the metal and its ore and give the reaction involved.
Answer:
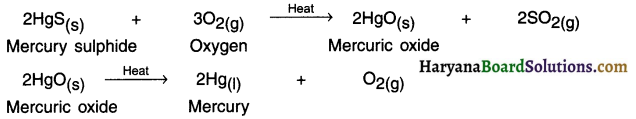
1. The only metal that exists in liquid state is mercury (Hg). Mercury is obtained from the sulphide ore called cinnabar ore (HgS).
2. Mercury sulphide (HgS) also known as cinnabar is an ore of mercury. On heating it, it first gets converted into mercuric oxide (HgO). On heating (HgO), we can obtain mercury (Hg) in pure form.
Question 64.
Compound X and aluminium are used to join railway tracks. (a) Identify the compound X, (b) Name the reaction and (c) Write down its reaction.
Answer:
(a) Compound X is iron (lll) oxide, Fe2O3.
(b) The reaction is known as a thermite reaction.
(c) The reaction that takes place is as follows

![]()
Question 65.
Switching over to alloys is an effective way to prevent corrosion. Give reason.
Answer:
1. Metals like iron, copper, etc. start getting corroded when they come in contact with air and water.
2. We can improve the anticorrosion properties of the metals by mixing them with certain other metals or non-metals.
3. For example, stainless steel which is an alloy made up of iron, chromium and nickel does not get affected by air, water or alkali and so it does not corrode.
4. Similarly, brass alloy which is made up of copper and zinc prevents corrosion in copper.
5. Thus, switching over to alloys is an effective way of preventing corrosion.
Question 66.
Explain the following
(a) Reactivity of Al decreases if it is dipped in HNO3
(b) Carbon cannot reduce the oxides of Na or Mg
(c) NaCl is not a conductor of electricity in solid state whereas it does conduct electricity in aqueous solution as well as In molten state
Answer:
(a) Nitric acid (HNO3) is an oxidizing agent. When aluminium is dipped in HNO3, an oxide layer of aluminium is formed on the surface of the metal. This prevents it from further reaction. As a result, reactivity of Al decreases.
(b) Na, Mg. etc., metals are quite reactive and are present towards the top portion of the reactivity series. As a result, these metals have more affinity with oxygen than carbon. Therefore, their oxides are stable. To reduce them with carbon, very high temperature is required and at that temperature they will form their corresponding carbides. Hence, their oxides cannot be reduced by carbon.
(c) One of the conditions for conduction of electricity is movement of ions. Ions of solid or dry NaCl cannot move to carry the charge. However, they are free in molten state and in aqueous solution to carry the charge and hence, NaCl conducts electricity in molten state.
Very Short Answer Type Questions
Question 1.
What are metals?
Answer:
The elements which lose electrons to become positively charged Ions are called metals.
Question 2.
State two physical properties of metals.
Answer:
Metals conduct heat and electricity. They are hard, shiny, heavy and sonorous (i.e. make sound on banging).
Question 3.
Define: Thermal conductivity
Answer:
The ability of a material to conduct heat from one end to another is called thermal conductivity.
Question 4.
What is sonority?
Answer:
The characteristic, of metal to produce a deep resonant sound on striking is called the property of sonority.
![]()
Question 5.
What makes aluminium and copper the ideal metals for making wires?
Answer:
Both aluminium and copper are easily available, cheap, conduct electricity and do not rust. Hence
Question 6.
Name two metals that do not react with oxygen.
Answer:
Gold and silver
Question 7.
What are non-metals?
Answer:
Elements that form negative ions by gaining electrons are called non-metals.
Question 8.
State two physical properties of non-metals.
Answer:
Non-metals are dull aid are poor conductor of heat and electricity.
Question 9.
How can you say that magnesium oxide is basic?
Answer:
The solution of MgO turns red litmus paper blue. This suggests that
Question 10.
What is an allotrope?
Answer:
Some elements possess the property of existing in two or more different forms in the same physical state. Such a property is called allotropy. The different forms of the elements are called allotropes.
Question 11.
A non-metal exists in two different forms Y and Z. Y Is the hardest natural substance, whereas Z is a good conductor of electricity Identify Y and Z.
Answer:
Y is diamond whereas Z is graphite.
Question 12.
Name the metal that has such a less melting point that It can melt with the heat of your palm.
Answer:
Gallium
Question 13.
Which metal oxides dissolve In water to form alkali? Write their balanced chemical equations.
Answer:
Oxides of reactive metals such as sodium and potassium dissolve in water to form alkali.
Reactions:
Na2O(s) + H2O(l)→ 2NaOH(aq)
K2O(s) + H2O(l) → 2KOH(aq)
Question 14.
Give chemical reaction when an iron strip is put in the solution of copper sulphate.
Answer:
Fe + CuSO4 → FeSO + Cu
![]()
Question 15.
What happens when metals react with acids?
Answer:
When metals react with acids they give salt and produce hydrogen gas.
Question 16.
Which metal out of calcium, aluminium, iron and zinc will perform the fastest reaction with acid to liberate hydrogen gas? Arrange the metals in the descending order with respect to the rate of liberation of hydrogen gas.
Answer:
1. Out of the given elements, calcium will liberate hydrogen gas at the fastest rate followed by aluminium, zinc and iron.
2. Arrangement of metals: Ca > Al > Zn > Fe.
Question 17.
Why copper does not react with dilute hydrochloric acid (HCl)?
Answer:
Since copper is not an active metal like Na, K.Zn. etc. it does not react with dilute hydrochloric acid (HCl).
Question 18.
Name two metals which react with dilute HNO3 to evolve hydrogen gas.
Answer:
Manganese (Mn) and magnesium (Mg)
Question 19.
What is the reactivity (activity) series of metals?
Answer:
The arrangement of metals in decreasing order of their reactivities is called the reactivity series of metals
Question 20.
How can you call a metal as more reactive metal?
Answer:
If a metal loses electrons rapidly it forms a positive ion and reacts quickly with other substance such metal is then termed as a more reactive metal.
Question 21.
The following reaction takes place when aluminium powder is heated with MnO2
3MnO2(s) + 4Al(s) → 3Mn(l) + 2Al2O3(l) + Heat
(a) Is aluminium getting reduced?
(b) Is MnO2 getting oxidized?
Answer:
(a) No, aluminium is not getting reduced. it is getting oxidized because there is addition of oxygen.
(b) No, MNO2 is getting reduced because there is removal of oxygen.
Question 22.
What is an ion?
Answer:
An ions an electrically charged atom (or group of atoms)
Question 23.
How is an ion formed?
Answer:
When an atom gains or loses electrons, it forms an ion.
![]()
Question 24.
What is cation? OR How is positive ion formed?
Answer:
If an element has 1,2 or 3 electrons in the outermost shell of its atoms, then it loses these electrons and forms a positively charged ion or cation.
Question 25.
What is anion? OR How is negative Ion formed?
Answer:
If an element has 5.6 or 7 electrons In the outermost shell of its atoms, then it gains these electrons and forms a negatively charged ion or anion.
Question 26.
Define: ionic bond.
Answer:
The chemical bond formed by the transfer of electrons from one atom to another is known as an ionic bond.
Question 27.
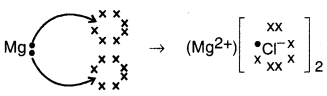
Show how electrons transfer in the formation of MgCl2 from Its elements.
Answer:
The transfer of electron in the formation of MgCl2 from its elements is shown below:
Question 28.
How do metals and non-metals combine?
Answer:
Metals and non-metals combine through the transfer of electrons from metals to non-metals to form ionic bonds.
Question 29.
What is gangue?
Answer:
Impurities such as soil, sand, etc. present in the ore is called gangue.
Question 30.
What is concentration of ore?
Answer:
The removal of impurities from the ore is called concentration of ore?
Question 31.
What is mineral?
Answer:
The natural materials in which the metals or their compounds are found in earth are called minerals.
Question 32.
What is ore?
Answer:
Those minerals from which the metals can be extracted conveniently and profitably are called ores.
Question 33.
What is roasting?
Answer:
Roasting is the process in which sulphide ore is heated strongly in the presence of air to convert it into metal oxide.
Question 34.
What is calcination?
Answer:
Calcination is the process in which carbonate ore is heated strongly in the absence of air to convert it into metal oxide.
![]()
Question 35.
What is rust?
Answer:
The rust-brown flaky layer that develops on iron when iron is exposed to moist air is called rust.
Question 36.
What is corrosion?
Answer:
The erosion reaction that takes place between a metal and water or moisture when they come in contact is called metallic corrosion.
Question 37.
Can rusting of Iron nail occur In distilled water?
Answer:
Distilled water does not contain dissolved oxygen. Hence, rusting will not occur in it.
Question 38.
Which two things are necessary for corrosion?
Answer:
Presence of air and water
Question 39.
What will you do to prevent corrosion on iron sheets used in the roof of the factories?
Answer:
I would either use galvanized sheets or paint the sheets.
Question 40.
What are the constituents of solder alloy? Which property of solder makes It suitable for welding electrical wires?
Answer:
Solder is an alloy made from 50% lead and 50% tin. Solder has a low melting point and so it is used for soldering i.e. welding electrical wires.
Fill in the Blanks:
1. …………………..is a liquid non-metal.
Answer:
Bromine
2. …………………….. and ………………… are two aliotropes of carbon.
Answer:
Diamond and graphite
![]()
3. ……………….. metals (type) are so soft that they can be cut with knife.
Answer:
Alkali
4. Most metals produce ………………… when dissolved in water.
Answer:
Basic oxides
5. When sodium dissolves in water it produces (name of compound).
Answer:
Sodium hydroxide
6. On heating copper, it gets coated with ……………
Answer:
Black coloured layer of copper (II) oxide
7. When calcium reacts with water it produces hydrogen but the hydrogen does not catch tire because ………………..
Answer:
The heat evolved is not sufficient enough to catch fire
8. Although when metal reacts with acids hydrogen gas is evolved, it is not the case when metal reacts with ……………. acid.
Answer:
Nitric
9. On putting pieces of copper in dilute HCl, neither bubbles are produced nor temperature changes. This means that copper …………………………..
Answer:
Does not react with dilute HCl
10. Ionic compounds are also called ……………….
Answer:
Electrovalent compounds
![]()
11. The most reactive metals are placed at the ………………….. in the reactivity series.
Answer:
Top
12. In a reactivity series, metals that lie above ………………. are more reactive than the metals that lie below it.
Answer:
H
13. Ores of many metals are found in the form of oxides because ………………
Answer:
Oxygen is very reactive metal and is found in abundance in the earth.
14. Highly reactive metals are mainly extracted with ………………… process.
Answer:
Electrolysis
15. A carbonate ore will undergo while a sulphide ore ………………. before oxidizing.
Answer:
Calcination; Roasting
16. Mercury ore is also called ………………
Answer:
Cinnabar
17. Metal carbonates and metal sulphide must be first reduced to their corresponding oxides because ………
Answer:
It is easier to obtain a metal from Its oxides.
18. Is used as an electrolyte in electrolysis of copper……………………
Answer:
Copper sulphate
19. The unit of for measuring the purity of gold is ………………
Answer:
Carat
![]()
20. What is common among magnesium, zinc and iron?
Answer:
They react with vapour to produce hydrogen gas
21. The most common reducing agent for reducing metal oxides is …………………………
Answer:
Carbon
22. Highly reactive metals such as sodium, calcium and aluminium are used as reducing agents because………………………
Answer:
They can displace metals of lower reactivity from their compounds
23. …………….. and ……………….. are reacted to join the railway tracks.
Answer:
Iron (III) oxide; aluminium
24. Carbon cannot reduce very reactive metals such as sodium, magnesium and aluminium because these metals ……….
Answer:
Have more affinity for oxygen rather than carbon
25. In electrolytic refining, the insoluble impurities ……………..
Answer:
Settle at the bottom of anode
26. The erosion reaction of a metal with water or moisture is called …………….
Answer:
Erosion
27. …………………… percentage of carbon added to iron makes it very strong and hard.
Answer:
0.05
28. Steel is formed by mixing ………………. to iron.
Answer:
Nickel and chromium
29. In an alloy, It one of the metals is mercury, the alloy will be known as ………………..
Answer:
Amalgam
![]()
True Or False
1. Although iodine is a non-metal, it is lustrous. — True
2. Metalloids are called so because they exhibit the properties of metals as well as non-metals. — True
3. Iron does not burn on heating but iron fillings burn vigorously when sprinkled in the flame. — True
4. Magnesium reacts vigorously with cold water. — False
5. Metals like aluminium, iron and zinc neither react with cold water, nor with hot nor with steam. — False
6. Ionic compounds have low melting and boiling points. — False
7. Ionic compounds have the ability to conduct electricity in their solid forms. — False
8. Electrovalent compounds are soluble in organic solvents such as petrol and kerosene. — False
9. In electronic refining, pure metal is taken as anode. — False