Haryana State Board HBSE 10th Class Science Important Questions Chapter 1 Chemical Reactions and Equations Important Questions and Answers.
Haryana Board 10th Class Science Important Questions Chapter 1 Chemical Reactions and Equations
Question 1.
What is physical and chemical change? List out few examples of both.
Answer:
(A) Physical change:
- Change in the physical properties of a substance is known as physical change.
- Colour, shape size, temperature, odour, appearance, etc. are all physical properties. Depending upon the process one or more of these properties may change.
Examples of physical change:
Melting of ice, Heating water, Breaking an object, Dissolving sugar/salt in water, etc.
![]()
(B) Chemical change:
When a substance combines with another substance such that one or more new product is formed through chemical reaction then such a change is called chemical change. In short, chemical change means chemical reaction.
Examples:
- Souring of milk when it is left at room temperature in summer
- Rusting of iron tawa or iron items when exposed to a humid atmosphere
- Fermenting grapes for making wine
- Cooking food
- Digestion of food
In all these cases the nature and identity of original substances change due to occurrence of chemical reaction.
Question 2.
How can one find out if a chemical reaction has taken place?
Answer:
If one or more following changes are observed in a process then we can say that a chemical reaction has taken place:
- Gas has evolved
- Formation of precipitation
- Change in colour
- Change in state
- Change in temperature
Question 3.
What is a chemical reaction and a chemical equation? How does chemical equation gives idea about atoms, molecules and elements present in it?
Answer:
Chemical reaction :
1. A process in which one or more reactants are chemically. changed into one or more new products is known as a chemical reaction.
2. A chemical equation is the symbolic representation of a chemical reaction.
![]()
Chemical equation :
1. The chemical formula of a compound gives the chemical composition of atoms and molecules of the elements present there in.
2. Thus, a chemical equation represents the types of atoms of an element and their numbers present in the compounds involved.
Examples :
- Water molecule is expressed as H2O. Here, H represents hydrogen whereas O represents oxygen.
- The subscript 2 of H indicates that H2O is formed from 2 atoms of hydrogen and 1 atom of oxygen.
- Similarly, CH4 indicates that it is formed from 1 atom of carbon and 4 atoms of hydrogen.
Question 4.
What are reactants and products? Explain with an example.
Answer:
The elements/compounds that undergo chemical reaction are called reactants while those produced during the reaction are called products. Example: When magnesium is burnt in oxygen it gets converted to magnesium oxide.
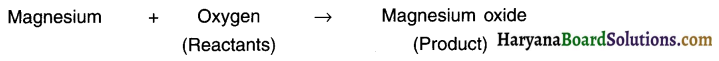
Question 5.
What is descriptive-equation?
Answer:
An equation described using sentences is called a descriptive-equation or sentence-equation.
Example: When magnesium ribbon is burnt in oxygen, it gets converted to magnesium oxide.
Question 6.
What is a word-equation?
Answer:
1. Writing a chemical equation in the form of words i.e. name of reactants and products is called word-equation.

2. The reactants are written on left hand side (L.H.S.) with plus sign between reactants. Products are written on Right Hand Side (R.H.S.) with a plus sign between products.
3. The arrow head points towards the products and shows the direction of the reaction i.e. formation of products from reactants.
![]()
Question 7.
What is a chemical equation?
Answer:
1. The method of representing a chemical reaction using symbols and formulae of substances involved (i.e. reactants and products) is known as a chemical equation. Example: When a magnesium ribbon is burnt in oxygen, it gets converted to magnesium oxide.
2. Magnesium will be represented as Mg’, oxygen as O2 and magnesium oxide as ‘MgO’. Thus, the chemical equation is:
Mg + O2 → MgO
3. This is just a skeletal chemical equation, It needs to be balanced.
(Note: This chemical equation is unbalanced. The correct chemical equation after balancing would be : Mg + O2 → 2MgO)
Question 8.
What is balancing a chemical-equation? Why is it necessary to balance It?
Answer:
The process of adding atoms to the elements on one or both sides i.e. reactant and product side so that the numbers of atoms of elements on each side becomes equal is known as balancing a chemical equation. Such an equation is called a balanced equation.
Need of balancing:
1. As per the universal law of conservation of mass, mass can neither be created nor destroyed in a chemical reaction. In other words, the total mass of the elements present in the products of chemical reaction should be equal to the total mass of the elements present in the reactants.
2. Now atoms have mass. Hence. in order to fulfill the above condition we need to make sure that the number of atoms in each element remains same on both the sides.
(a) Mg + O2 → MgO Unbalanced. Hence, wrong chemical equation
(b) 2Mg + O2 → 2MgO ….. Balanced. Hence, correct chemical equation
Question 9.
Mention steps for balancing a chemical equation giving an example of reaction of heated iron with steam. Consider the following example:
1. When heated iron metal reacts with steam, it forms iron oxide and hydrogen.
Skeletal equation:
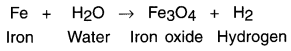
Steps for balancing the above equation:
Step 1:
Take a note of each molecule on left hand side and right hand side and count the number of atoms in each.
|
Element Present |
No. of atoms in reactants (LHS) |
No. of atoms in Products (RHS) |
|
Fe |
1 |
3 |
|
H |
2 | 2 |
| O | 1 |
4 |
Step 2:
1. Ideally one should start balancing an equation from the most complex compound Le. the one that has maximum number of atoms. Moreover, one can start balancing from any side i.e. reactant side or product side. In the selected compound, select the element that contains maximum number of atoms.
![]()
2. As we can see here, the most complex compound of the equation is Fe3O4 and the element in it with maximum number of atoms is oxygen. So, we start balancing one equation by first balancing oxygen on L.H.S. and R.H.S.
To balance oxygen atoms:
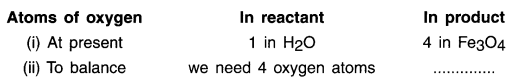
3. We cannot alter the formulae of a compound to equalize the number of atoms. For example, to balance oxygen atoms we cannot add 4 atoms of oxygen to H2O as H2O4 or (H2O)4 or (H24O).
4. The correct way is to put a co-efficient number to the compound. We take the co-efficient ‘4’ and put in into H2O to make it 4H2O.
The reaction now becomes (To balance hydrogen atoms): Fe + 4H2O → Fe3O4 + H2
(Note that oxygen became 4 in reactant but now hydrogen became 8)
Step 3:
1. Both ‘Fe’ and ‘H’ are unbalanced but, the most complex compound i.e. the compound with highest number of atoms is 4H2O. So we will balance hydrogen atoms first.
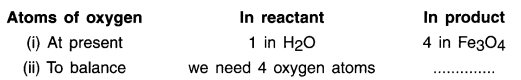
The new equation is: Fe + 4H2O → 4 Fe3O4 + 4H2
Step 4:
By looking at the above equation we can see that atoms of all except ‘Fe’ are balanced
To balance Fe:

The final reaction then is : 3Fe + 4H2O → Fe3O4 + 4H2
Step 5:
Finally to check the correctness of the equation balanced, we count atoms of each element on both the sides of the equation.
| No. of atoms | In reactants (LHS) | In Products | Status |
| Fe | 3 | 3 | Balanced |
| H | 8 | 8 | Balanced |
| O | 4 | 4 | Balanced |
The equation is now balanced
Step 6:
1. Although the equation is now balanced it does not tells us in which state do reactants react and products emerge. So, the final step involves putting signs of states of elements/compounds involved.
(Note: It is not necessary to show state unless specified.)
![]()
2. The solid, liquid, aqueous and gaseous forms are represented as (s), (I), (aq) and (g) respectively.
The word aqueous (aq) means a compound is present as solution in water.
The final equation along with states is: 3Fe(S) + 4H2O(g) → Fe3O4(s) + 4H2(g)
3. In H2O, the symbol (g) is used. This means that water is present in the form of steam.
Question 10.
How can a chemical equation be represented more informatively?
A chemical equation can be made more Informative in following three ways:
(a) By indicating physical state of reactants and products.
We can mention the states of reactants and products namely solid (s), liquid (I), aqueous (aq) and gaseous (g).
Example: Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
(b) By indicating ‘change In heat’ that takes place in the reaction.
Example:
1. When carbon reacts with oxygen, carbon dioxide is formed and heat is released.
C(s) + O2(g) → CO2(g) + Heat
2. In this reaction heat or heat energy is released. Hence, the reaction tells us that burning of carbon in oxygen is an exothermic i.e. heat releasing reaction.
(c) By indicating the conditions under which the reaction takes place.
Sometimes, the reaction takes place under conditions such as sunlight, atmospheric pressure, catalysts. Mentioning such conditions makes a reaction more informative.
![]()
Example:
The mixture of carbon monoxide and hydrogen gas is compressed under 300 atmospheric pressure and then passed over a catalyst zinc oxide and chromium oxide and heated to 300°C to form methanol (methyl alcohol)

Question 11.
Enlist ways of making a chemical equation more informative along with one example of each.
Answer:
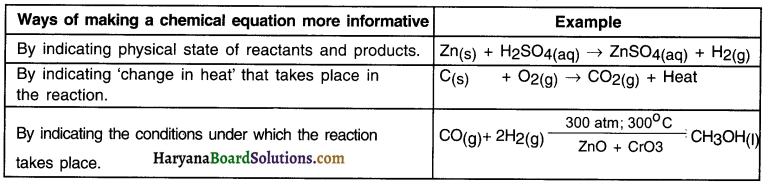
Question 12.
Write the skeletal equation for the following reactions:
(i) Hydrogen sulphide reacts with sulphur dioxide to form sulphur and water.
(ii) Methane on burning combines with oxygen to produce carbon dioxide and water.
Answer:
(i) H2S + SO2 → S + H2O
(ii) CH4 + O2 → CO2 + H2O
Question 13.
Balance the following skeletal equations:
(i) BaCl2 + H2SO4 → BaSO4 + HCl
(ii) FeCl2 + H2S → HCl + FeS
(iii) Fe + H2O → Fe3O4 + H2
(iv) NH3 + CuO → Cu + N2 + H2O
Answer:
(i) BaCl2 + H2SO4 → BaSO + 2HCl
(ii) FeCl2 + H2S → 2HCl + FeS
(iii) 3Fe + 4H2O → Fe3O4 + 4H2
(iv) 2NH3 + 3CuO → 3Cu + N2 + 3H2O
Question 14.
It is necessary to balance a chemical reaction. Give reason.
Answer:
1. In a chemical reaction, atoms are neither created nor destroyed but, just exchanged.
2. Thus, the number of atoms of the reactants and products should remain same.
3. Moreover, as per the law of conservation, the chemical equation must be balanced.
4. Hence, it is necessary to balance a chemical reaction.
![]()
Question 15.
Enlist few important types of chemical reactions.
Answer:
Types of chemical reactions:
(1) Combination reaction
(2) Decomposition reaction
(3) Displacement reaction
(4) Double displacement reaction
(5) Oxidation and reduction reaction
Question 16.
What is combination reaction? Explain with the help of example of calcium oxide.
Answer:
Combination reaction:
A reaction in which two or more substances (elements or compounds) combine to form a single substance is called combination reaction.
Example: When calcium oxide and water are mixed, calcium oxide reacts vigorously with water to produce slaked lime (calcium hydroxide) and release a large amount of heat.

Here, calcium oxide and water combined to produce a single product, calcium hydroxide.
Question 17.
State two examples of combination reaction that take place in presence of oxygen.
Answer:
Since oxygen is added in these reactions, these are also called oxidation reactions.
![]()
Question 18.
What is an exothermic reaction? State one example.
Answer:
A chemical reaction in which heat is released (evolved) along with the formation of products is called an exothermic reaction. (Note: Exothermic is not a major type of reaction unlike combination reaction, displacement reaction, etc.)
Example:
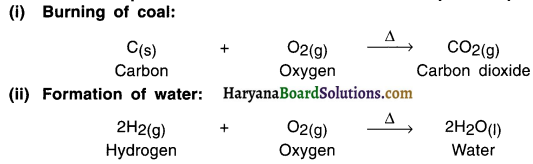
(i) Burning of natural gas —

(ii) Respiration
(iii) Decomposition of vegetable matter into compost.
Question 19.
What is endothermic reaction? Give example.
Answer:
Endothermic reaction: A reaction in which heat is absorbed or say required is called endothermic reaction.
Example: 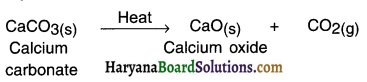
When calcium carbonate is supplied heat, calcium oxide and carbon dioxide are formed.
Question 20.
Why is respiration considered exothermic reaction? Explain. OR Explain how food helps in respiration with the help of chemical reaction.
Answer:
1. Food gives us energy which helps us to survive.
2. When we eat food, our body starts digestion process. During digestion the food gets broken into simple substances. For example, carbohydrate present in rice, potato, etc. breaks down to form glucose.
3. The glucose then combines with oxygen present in the body cells and provide energy. This reaction is known as respiration.
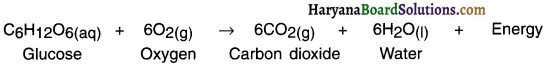
Since heat energy is released during respiration, it is known as exothermic reaction.
Question 21.
What is decomposition reaction? State its types.
Answer:
Decomposition reaction :
1. A reaction in which a single reactant (substance) breaks down i.e. decompose to form two or more substances is called decomposition reaction.
2. Decomposition reaction is opposite reaction of combination reaction.
3. To decompose a compound, heat, electric current, light, etc. are supplied during the decomposition reaction.
Type of decomposition reaction:
- Thermal decomposition,
- Electrical decomposition,
- Light decomposition reaction.
![]()
Question 22.
Define thermal decomposition, giving example of lime stone.
Answer:
Thermal decomposition : The decomposition reaction done by supplying heat is known as thermal decomposition reaction.
Example :
When lime stone (calcium carbonate) is heated, it decomposes to give calcium oxide and carbon dioxide.
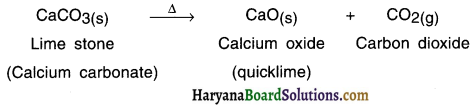
Question 23.
What is electrical decomposition? Explain with the help of an example.
Answer:
Electrical decomposition reaction (Electrolysis) :
1. The decomposition reaction done by supplying electric current is known as electrical decomposition reaction or electrolysis reaction.
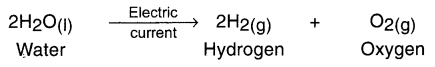
2. For example, by adding one or two drops of sulphuric acid to water and supplying Direct Current (DC) to it, its electrical decomposition takes place and it decomposes into hydrogen and oxygen.
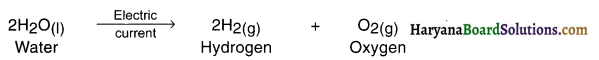
Electrolysis reaction can be studied through an instrument called voltameter.
Question 24.
What happens when silver bromide is exposed to light? OR State and explain light decomposition reaction with the help of an example.
Answer:
1. When silver bromide is exposed to light it decomposes to form silver metal and bromine vapour.
2. The decomposition of silver bromide took place in the presence of light and hence the reaction is light decomposition reaction.
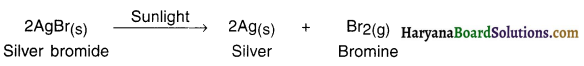
Question 25.
State and explain types of decomposition reaction.
Answer:
Thermal decomposition : The decomposition reaction done by supplying heat is known as thermal decomposition reaction.
Example :
When lime stone (calcium carbonate) is heated, it decomposes to give calcium oxide and carbon dioxide.

Electrical decomposition reaction (Electrolysis) :
1. The decomposition reaction done by supplying electric current is known as electrical decomposition reaction or electrolysis reaction.
2. For example, by adding one or two drops of sulphuric acid to water and supplying Direct Current (DC) to it, its electrical decomposition takes place and it decomposes into hydrogen and oxygen.
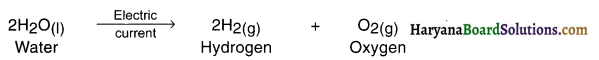
Electrolysis reaction can be studied through an instrument called voltameter.
![]()
1. When silver bromide is exposed to light it decomposes to form silver metal and bromine vapour.
2. The decomposition of silver bromide took place in the presence of light and hence the reaction is light decomposition reaction.
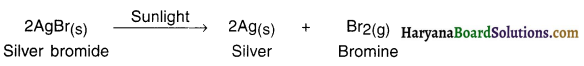
Question 26.
What is a displacement reaction? State its types.
Answer:
When a more reactive element displaces (removes) less reactive element from its compound it is called displacement reaction.
Types:
(a) Single displacement reaction (or simply displacement) and (b) Double displacement reaction
Question 27.
What is displacement (or single displacement) reaction? Give two examples.
Answer:
In single displacement reaction, the more reactive element reacts with a compound and takes the place of another element i.e. displaces (or remove) the less reactive element.
Example:
(1) 
Iron is more reactive than copper. Hence, when iron reacts with solution of copper sulphate, it removes (or displaces) copper and gets attached to sulphate.
(2) 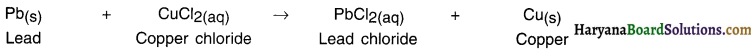
Lead which is more reactive than copper when reacts with copper chloride displaces copper and forms lead chloride.
Question 28.
Explain displacement reaction of zinc with copper sulphate.
Answer:
1. Zinc is more active metal than copper. Hence, when zinc strip is kept in copper sulphate solution, it displaces copper fram copper sulphate and forms zinc sulphate and copper.
2. The blue colour of copper sulphate fades and the solution becomes colourless.

Question 29.
What is double displacement reaction? Give one example.
Answer:
1. A reaction in which two different ions of group of atoms in the reactant molecule are displaced by each other is called double-displacement reaction.
2. A white substance insoluble in water is formed as precipitate and hence the reaction is also called precipitation reaction.
Example:
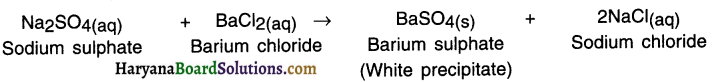
3. When the aqueous solution of sodium sulphate reacts with aqueous solution of barium chloride a white precipitate of BaSO4 is formed.
4. In this double displacement reaction, sulphate ions \(\mathrm{SO}_4^{2-}\) are displaced by chloride ions Cl– and vice-versa. Thus, double displacement shows exchange of ions.
Question 30.
How does ion exchange take place in a double-displacement reaction?
Example:
Answer:
When silver nitrate solution is added to sodium chloride solution, then a white precipitate of silver chloride is formed along with sodium nitrate solution.
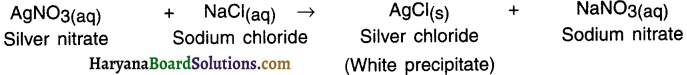
Ion exchange:
1. In this reaction, silver ions (Ag+) of silver nitrate react with chloride ions (Cl–) of sodium chloride to form a new compound called silver chloride (Ag+Cl– or simply AgCl).
2. Sodium ions (Nat) of sodium chloride react with nitrate ions (NO3–) of silver nitrate to form sodium nitrate
(Na+NO– or NaNO3).
![]()
Question 31.
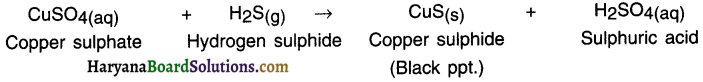
State the double displacement reaction of hydrogen sulphide gas with copper sulphate solution.
Answer:
When hydrogen sulphide gas is passed through copper sulphate solution, black precipitate of copper sulphide is formed along with sulphuric acid solution.
Question 32.
Define and very briefly explain oxidation reaction, reduction reaction and redox reaction.
Answer:
Oxidation reaction :
In a chemical reaction, if oxygen is added to or hydrogen is removed from an element, molecule or a compound, it is called an oxidation reaction.
Reduction:
- To every oxidation reaction, simultaneously there occurs a reduction reaction.
- The opposite reaction of oxidation reaction in which hydrogen is added to or oxygen is removed from an element, molecule or a compound is called reduction.
Redox:
In a redox reaction, oxidation and reduction reactions take place simultaneously. Hence, the entire reaction is called Redox (Red = reduction, ox = oxidation) reaction.
Oxidation and reduction can be understood with a simple table:

Question 33.
What is a redox reaction? Explain with examples. OR Explain briefly oxidation and reduction reactions.
Answer:
Oxidation reaction :
In a chemical reaction, if oxygen is added to or hydrogen is removed from an element, molecule or a compound, it is called an oxidation reaction.
For Example:
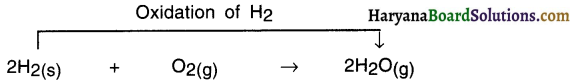
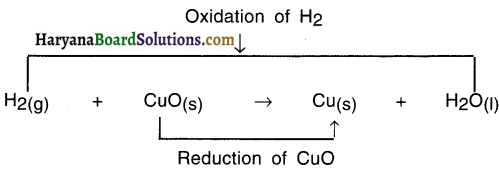
In this reaction, oxygen is added and so hydrogen gets oxidized. Hence, it is an oxidation reaction.
Reduction reaction :
- To every oxidation reaction, simultaneously there occurs a reduction reaction.
- The opposite reaction of oxidation reaction n which hydrogen is added to or oxygen is removed from an element, molecule or a compound is called reduction.
For Example:
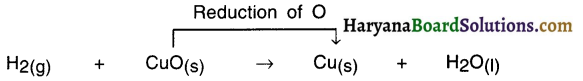
In this reaction, oxygen is removed from CuO and so it is a reduction reaction.
![]()
Redox reaction:
Since reduction reaction and oxidation occur simultaneously, the reaction is called redox
(Note: Reduction Red and Oxidation = Ox. Thus, redox reaction.)
For Example:
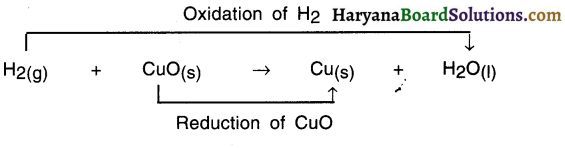
In this reaction —
- HO is formed from H2 Since H2 got added to Oxygen O. So it is an oxidation reaction,
- CuO got converted into Cu because oxygen got removed and so it is reduction reaction.
- Since oxidation and reduction have taken place simultaneously, this whole reaction is called a redox reaction.
Question 34.
State two redox reactions.
ZnO + C → Zn + CO
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
In first reaction carbon is oxidized to CO and ZnO is reduced to Zn. In second reaction HCl is oxidized to Cl2 whereas MnO2 is reduced to MnCl2.
Question 35.
Define different types of chemical reactions and give one example of each.
Answer:
Types of chemical reactions:
1. Combination reaction:
A reaction in which two or more substances (elements or compounds) combine to form a single substance is called combination reaction.
Example:
When calcium oxide and water are mixed, calcium oxide reacts vigorously with water to produce slaked lime (calcium hydroxide) and release a large amount of heat.

2. Decomposition reaction: A reaction in which a single reactant (substance) breaks down i.e. decompose to form two or more substances is called decomposition reaction.
Types:
(a) Thermal decomposition :
Example: When lime stone (calcium carbonate) is heated, it decomposes to give calcium oxide and carbon dioxide.

(b) Electrical decomposition reaction (Electrolysis) :
Example: Adding one or two drops of sulphuric acid to water and supplying Direct Current (DC) to it, its electrical decomposition takes place and it decomposes into hydrogen and oxygen.
(c) Light decomposition reaction:
Example: When silver bromide is exposed to light it decomposes to form silver metal and bromine vapour.
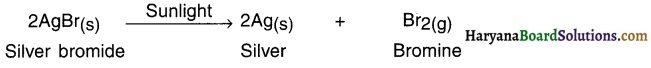
3. Displacement reaction:
When a more reactive element displaces (removes) less reactive element from its compound it is called displacement reaction.
Types:
(a) Single displacement reaction (or simply displacement):
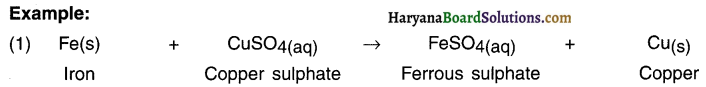
Iron is more reactive than copper. Hence, when iron reacts with solution of copper sulphate, it removes (or displaces) copper and gets attached to sulphate.
(b) Double displacement reaction:
Example:

When the aqueous solution of sodium sulphate reacts with aqueous solution of barium chloride a white precipitate of BaSO4 is formed.
![]()
4. Redox reaction:
The reaction in which reduction reaction and oxidation reaction occur simultaneously is called redox reaction
Question 36.
What is corrosion? Write a brief note.
Answer:
1. When a metal comes in contact with humid air, moisture or a chemical such as acid, the surface of metal starts getting eaten up. This is called corrosion.
2. Corrosion is mainly caused by the oxidation of metals in humid air. Rusting of iron is the most common form of corrosion.
3. Due to corrosion, silver ornaments become black and a green coating gets deposited on copper vessels.
4. Corrosion damages vehicles, bridges, iron rails, ships and other metal structures.
5. Corrosion of iron is a serious problem. It causes huge sum to be spent on maintaining iron structures and replacing corroded parts.
Question 37.
What is rancidity?
Answer:
1. Oxidation affects food that contains fats and oils.
2. When food items (such as snacks like pun, chakni, chavana, etc) prepared using fat and oils are kept for longer period, they develop an unpleasant smell and taste. We then say that the food item has become rancid.
3. One can reduce the rate of rancidity by keeping the food items in air-tight containers. This, slows down oxidation of food.
4. Packets of chips are flushed with nitrogen to prevent chips from becoming rancid.
Question 38.
Explain the following terms with one example each. (a) Corrosion, (b) Rancidity.
Answer:
Types of chemical reactions:
1. Combination reaction:
A reaction in which two or more substances (elements or compounds) combine to form a single substance is called combination reaction.
Example:
When calcium oxide and water are mixed, calcium oxide reacts vigorously with water to produce slaked lime (calcium hydroxide) and release a large amount of heat.

2. Decomposition reaction: A reaction in which a single reactant (substance) breaks down i.e. decompose to form two or more substances is called decomposition reaction.
![]()
Types:
(a) Thermal decomposition :
Example: When lime stone (calcium carbonate) is heated, it decomposes to give calcium oxide and carbon dioxide.

(b) Electrical decomposition reaction (Electrolysis) :
Example: Adding one or two drops of sulphuric acid to water and supplying Direct Current (DC) to it, its electrical decomposition takes place and it decomposes into hydrogen and oxygen.

(c) Light decomposition reaction:
Example: When silver bromide is exposed to light it decomposes to form silver metal and bromine vapour.
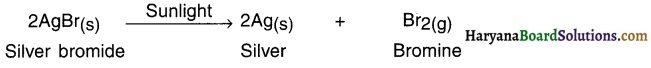
3. Displacement reaction: When a more reactive element displaces (removes) less reactive element from its compound it is called displacement reaction.
Types:
(a) Single displacement reaction (or simply displacement):

Iron is more reactive than copper. Hence, when iron reacts with solution of copper sulphate, it removes (or displaces) copper and gets attached to sulphate.
(b) Double displacement reaction:
Example:

When the aqueous solution of sodium sulphate reacts with aqueous solution of barium chloride a white precipitate of BaSO4 is formed.
4. Redox reaction:
The reaction in which reduction reaction and oxidation reaction occur simultaneously is called redox reaction

1. When a metal comes in contact with humid air, moisture or a chemical such as acid, the surface of metal starts getting eaten up. This is called corrosion.
2. Corrosion is mainly caused by the oxidation of metals in humid air. Rusting of iron is the most common form of corrosion.
3. Due to corrosion, silver ornaments become black and a green coating gets deposited on copper vessels.
4. Corrosion damages vehicles, bridges, iron rails, ships and other metal structures.
5. Corrosion of iron is a serious problem. It causes huge sum to be spent on maintaining iron structures and replacing corroded parts.
![]()
Question 39.
Differentiate between exothermic reaction and endothermic reaction.
| Exothermic reaction |
Endothermic reaction |
| 1. A chemical reaction in which heat is released (evolved) along with the formation of products is called an exothermic reaction. 2. For example, burning of natural gas, respiration, etc. |
1. A reaction in which heat is absorbed or say required is called endothermic reaction. 2. For example, reaction of calcium oxide with water. |
Question 40.
Give examples of each characteristic change that determine occurrence of a chemical reaction.
| Example | Change in characteristic |
| 1. When zinc reacts with sulphuric acid, bubbles of hydrogen gas are produced. | Here, gas has evolved. Also, temperature rises. |
| 2. Adding potassium iodide solution to solution of lead nitrate forms a yellow precipitate of lead iodide | Formation of precipitation. Also, colour changes to yellow |
| 3. When citric acid is added to purple coloured potassium permanganate, the solution becomes colourless. | Change in colour |
| 4. When water is added to quick lime, slaked lime is formed and a lot of heat energy is released. | Change in temperature |
| 5. Burning of candle wax produces water and carbon dioxide | Change in state of substance from solid to liquid when it undergoes combustion reaction. |
Question 41.
Balance the following equations.
KMnO4 + HCl → KCl + MnCl2 + H2O + Cl2
Balanced equation:
2KMnO4+ 16HCl → 2KCl + 2MnCl2 + 8H4O(l) + 5Cl2
Question 42.
Explain how calcium oxide gets converted into slaked lime and how slaked lime gets converted into calcium carbonate?
Answer:
1. When calcium oxide or say quick lime (CaO) is dissolved in water, solution of calcium hydroxide i.e. slaked lime (Ca(OH)2) is produced.
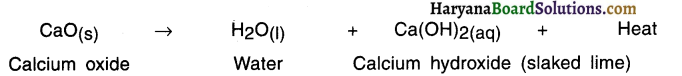
2. Calcium hydroxide is filtered and the filtered solution is applied to the walls as white wash.
3. On applying to the walls, calcium hydroxide reacts with the carbon dioxide (CO2) of the air and forms insoluble white thin layer of calcium carbonate (CaCO3) on the walls.

Question 43.
What happens when carbon dioxide and water react in the same ratio?
Answer:
When six molecules of carbon dioxide and six molecules of water undergo reaction, glucose is formed along with with evolution of oxygen gas.

Question 44.
State the respiration reaction.
Answer:
When food glucose. combines with oxygen present in the body cells, it gives energy to the body through respiration process.
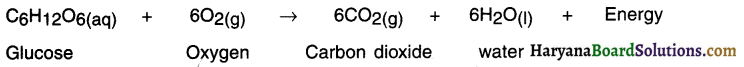
Question 45.
State the decomposition reaction of sodium chloride. OR State the electrolysis reaction of sodium chloride.
Answer:
When electric current is passed through molten sodium chloride, it undergoes electrolysis and decomposes into sodium metal and chlorine gas.
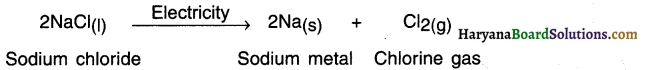
Question 46.
What happens when lead is placed in copper chloride solution?
Answer:
When a strip of lead (Pb) is p)aced in copper chloride (CuCl2) solution, more active lead displaces copper from the solution.
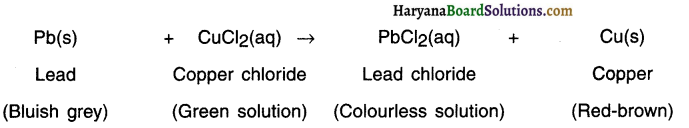
Question 47.
Magnesium is a reactive metal. What will happen if you put it in hydrochloric acid solution?
Answer:
Magnesium metal will react with hydrochloric acid to form magnesium chloride and hydrogen gas.

In this displacement reaction, magnesium displaces hydrogen from hydrochloric acid solution. This displacement reaction occurs because magnesium is more reactive than hydrogen.
Question 48.
How can the black coating of copper oxide be removed chemically?
Answer:
The black coating of copper oxide can be removed chemically by passing hydrogen gas over heated copper oxide. The black coating will turn brown in colour since oxygen will be removed by hydrogen.
CuO+H2 → Cu+H2O
Question 49.
State the ascending order of reactivity for metals Cu, Ag and Fe on the basis of reactions given below:
Answer:
(i) Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
(ii) Cu(s) + FeSO4(aq) → No reaction
(iii) Cu(s) + 2AgNO3(aq) → Cu(NO3)(aq) + 2Ag(s)
(iv) 2Ag(s) + Cu(NO3)2 → No reaction
Based on the given reactions, ascending order of reactivity of Cu, Ag and Fe is – Ag < Cu < Fe
![]()
Question 50.
State the reaction when iron (III) oxide is heated with aluminum powder. Also state the type of reaction.
Answer:
When iron (III) oxide is heated with aluminium powder, aluminium oxide and iron metal is formed.
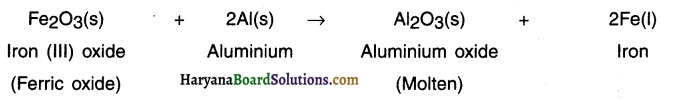
This is a displacement reaction. In this displacement reaction, a more reactive metal, aluminium displaces a less reactive metal, iron, from its oxide, iron (III) oxide.
Question 51.
Three test tubes are taken and marked as ‘X’, ‘Y’ and ‘Z’. In test tube X, iron nail is dipped in water. In test tube Y, Iron nail is dipped in mixture of water and oil. In test tube Z, iron nail is added with dry CaCl2. In which test tube, the iron nail will rust? Why?
Answer:
The iron nail which is dipped in test-tube containing water i.e. test-tube X will rust.
Reason: It is a property of iron that when it comes in contact of moisture it starts rusting.
Question 52.
Why do most of the metal articles become dull, when left in open air?
Answer:
Metal articles left in an open air reacts with the gases or say the air of atmosphere. Such metals then form a layer of oxide compound on their surface. This makes the metal lose their luster and become dull.
Question 53.
Why is photosynthesis considered as an endothermic reaction?
Answer:
1. An endothermic reaction is one which requires energy to occur.
2. In photosynthesis reaction, sunlight acts as an energy. Plants use this energy to form glucose from carbon dioxide and water. Hence, this reaction is called an endothermic reaction.
Question 54.
When the guests arrived at Mrs. Sudha Murthy’s house her daughter Nitya got very excited.
Answer:
She offered lemonade to the guests to which they agreed. Nitya went into kitchen and gathered ingredients such as lemon, sugar, water, salt, etc. and started preparing the lemonade in a large copper vessel. When Mrs. Murthy came to kitchen she stopped Nitya from using a copper vessel and asked to use a steel vessel. Why do you think she asked Nitya to do so?
- Lemon is acidic in nature, It contains citric acid.
- Although citric acid of lemon does not cause any harm but when it is put in vessel made of copper, it starts reacting with copper. This causes copper-poisoning in human body which leads to gastro-intestinal problems.
- Here, Nityas mother displayed value of awareness and concern about social health.
Question 55.
A student mixed solutions of lead (II) nitrate and potassium Iodide.
(a) Can you tell the colour of precipitate formed?
(b) Which type of chemical reaction is this? Provide your reason.
Answer:
(a) The precipitate formed is of yellow colour.
(b) The reaction is as follows:
Pb(NO3)2 + 2Kl → 2Pbl2 + KNO3
In the reaction, lead and potassium exchange their Ions and hence the reaction is a double displacement reaction.
Question 56.
Ritesh had a blue coloured salt in test-tube. The teacher did not tell the name of the salt. The teacher asked Ritesh to heat the salt. On heating it became white. Then the teacher asked him to add water, The salt again turned blue. Which salt did Ritesh have in the test-tube? State the reason for changes in colour.
Answer:
1. The substance is copper sulphate (CuSO2 . 5H2O). It is blue in colour.
2. On heating it loses water and so what remains is white coloured CUSO4.
3. On adding water it again becomes hydrated and regains blue colour.
Question 57.
Complete the missing components/variables given as x and y in the following reactions.
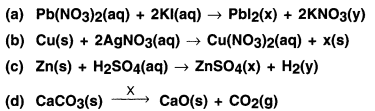
Answer:

Question 58.
Identify the reducing agent in the following reactions.
(a) 4NH3 + 5O2 → 4NO + 6H2O
(b) H2O + F2 → HF + HOF
Answer:
(a) In this reaction, NH3 is the reducing agent because it gives hydrogen and gets oxidized to NO.
(b) H2O is the reducing agent because the electronegative F gets added and so H2O gets oxidized to HOF.
![]()
Question 59.
Identify the oxidizing agent (oxidant) in the following reactions.
(a) Pb3O4 + 8HCl → 3PbCl2 + Cl2 + 4H2O
(b) 2Mg + O2 → 2MgO
Answer:
(a) HCl has been oxidized to Cl2 (Removal of H) and Pb3O4 has been reduced to PbCl2 (Removal of O). Hence, Pb3O4 is the oxidizing agent (oxidant).
(b) Mg has been oxidized to MgO (Addition of oxygen O). Hence, O2 is the oxidant.
Question 60.
Grapes hanging on the plant do not ferment but after being plucked from the plant can be fermented. Under what conditions do these grapes ferment? Is It a chemical or a physical change?
Answer:
1. When grapes are attached to the plant, they receive oxygen right upto the cell level. As a result, they undergo aerobic respiration and do not ferment.
2. When grapes are plucked, the oxygen does not reach the cell level and so the aerobic respiration does not occur.
3. Fermentation takes place only in the absence of oxygen I.e. under anaerobic condition. As a result, grapes start fermenting after being plucked.
Question 61.
During the reaction of some metals with dilute hydrochloric acid, following observations were made.
(A) Silver metal does not show any change
(B) The temperature of the reaction mixture rises when aluminium (Al) is added.
(C) The reaction of sodium metal is found to be highly explosive
(D) Some bubbles of a gas are seen when lead (Pb) is reacted with the acid
Explain these observations giving suitable reasons.
Answer:
(a) In the reactivity series, silver lies below hydrogen which means silver is less reactive than hydrogen. So, silver cannot displace hydrogen when reacted with acid.
(b) The reaction of Al with dilute HCl is exothermic i.e., heat is produced in the reaction. As a result, the temperature of the reaction mixture rises. The reaction is as follows.
2Al + 6HCl → 2AlCl3 + 3H2 + Heat
(c) Sodium is a very reactive metal. It reacts explosively (extremely rapidly) with hydrochloric acid to form sodium chloride and hydrogen along with the evolution of heat. H2 gas produced catches fire immediately.
(d) Lead is present just above the hydrogen in the activity series of metals. Hence, it is slightly more reactive and displaces hydrogen from acid very slowly that too upto a small extent. Hence, only bubbles of H2 are seen to be evolved.
Question 62.
Why do we store silver chloride in dark coloured bottles?
Answer:
Dark coloured bottles interrupt the path of light and prevent them from directly entering into the bottles. Storing silver chloride in dark coloured bottles does not allow the light to reach silver chloride in the bottles. This prevents its decomposition. If the silver chloride is not stored in dark bottles, the following reaction would take place.

Question 63.
A magnesium ribbon Is burnt in oxygen to give a white compound X accompanied by emission of light. If the burning ribbon is now placed in an atmosphere of nitrogen, it continues to burn and forms a compound Y.
(A) Write the chemical formulae of X and Y.
(B) Write a balanced chemical equation, when X is dissolved in water.
Answer:
The reaction for the first statement is:

(A) The chemical formulae of X and Y are: X = MgO; Y = Mg3N2
(B)
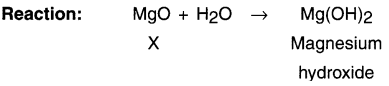
Very Short Answer Type Questions
Question 1.
What is physical change?
Answer:
Change in the physical properties of a substance is known as physical change.
![]()
Question 2.
What can change under physical change?
Answer:
Colour, shape, temperature, appearance or odour.
Question 3.
Give few examples of physical change.
Answer:
Melting of ice, heating water, breaking an object, dissolving sugar/salt in water.
Question 4.
What is chemical change?
Answer:
When a substance combines with another substance such that one or more new product is formed it is called chemical change. In short, chemical change is chemical reaction.
Question 5.
Give two examples of chemical change.
Answer:
(a) Rusting if iron,
(b) Souring of milk when it is left at room temperature for long.
Question 6.
Define reactants and products.
Answer:
The substances that undergo chemical reaction are called reactants while those produced during the reaction are called products.
Question 7.
What is word equation?
Answer:
Writing a chemical equation in the form of words i.e. name of reactants and products is called word-equation.
![]()
Question 8.
State one example of word equation.
Answer:
Hydrogen + chlorine = Hydrochloric acid
Question 9.
Define chemical equation.
Answer:
The method of representing a chemical reaction using symbols and formulae of substances involved (i.e. reactants and products) is known as a chemical equation.
Question 10.
State the law of conservation of mass.
Answer:
In a chemical reaction, mass can neither be created nor be destroyed. The total mass in the universe remains constant.
Question 11.
Write skeletal equation for: When heated iron metal reacts with steam, it forms iron oxide and hydrogen.
Answer:
Fe + H2O → Fe3O4 + H2
Question 12.
When you burn a silvery-white metal P, it burns with dazzling flame and produces white powder Q. What Is metal P and what is powder Q.
Answer:
Metal P is magnesium and white powder is magnesium oxide.
Question 13.
A chemical equation states 200 atm. on the arrow between LHS and RHS what does it mean?
Answer:
It means the reaction took place under 200 atmospheric pressure.
![]()
Question 14.
What is a catalyst?
Answer:
A substance that increases the rate of reaction without itself undergoing any permanent change is called a catalyst. For example, sunlight.
Question 15.
Match the following:
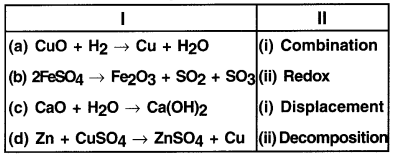
Answer:
(a – ii) (b – iv) (c – i) (d – iii)
Question 16.
Write the balanced chemical equation for the following:
Answer:
The mixture of carbon monoxide and hydrogen gas is compressed under 300 atmospheric pressure and then passed over a catalyst zinc oxide and chromium oxide heated to 300°C to form methanol (methyl alcohol)

Question 17.
What is a combination reaction?
Answer:
A reaction in which two or more substances combine to form a single substance is called combination reaction.
Question 18.
Balance: MnO2 + HCl → MnCl2 + Cl2 + H2O
Answer:
4 MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
Question 19.
Define exothermic reaction.
Answer:
A chemical reaction in which heat is released along with the formation of products is called an exothermic reaction.
![]()
Question 20.
State a chemical reaction for exothermic reaction.
Answer:
Burning of natural gas:
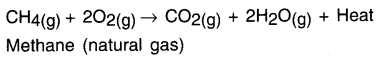
Question 21.
What is endothermic reaction?
Answer:
A reaction in which heat is absorbed or say required is called endothermic reaction.
Question 22.
Give an equation showing endothermic reaction.
Answer:
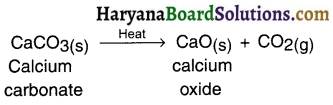
Question 23.
Classify the following chemical reactions into endothermic and exothermic.
(i) Electrolysis of water,
(ii) Burning of natural gas,
(iii) Decomposition of calcium carbonate and
(iv) Burning of magnesium ribbon in air.
Answer:
Exothermic reaction: (ii) and (iv),
Endothermic reaction: (I) and (iii)
Question 24.
Why does the colour of copper sulphate change when an ¡ron nail is dipped in it?
Answer:
Iron displaces copper from CuSO4 to form FeSO4 which is pale green in colour. Hence,
Question 25.
Define decomposition reaction.
Answer:
A reaction in which a compound splits into two or more simpler substances is called decomposition reaction.
Question 26.
State the types of decomposition reaction.
Answer:
(a) Electrical decomposition
(b) Thermal decomposition and
(c) Light decomposition.
Question 27.
When a white salt is heated it decomposes to produce brown fumes. Which is this salt?
Answer:
Lead nitrate Pb(NO3)2
![]()
Question 28.
If you strongly heat iron salt, its colour changes to brown along with emitting smell like burning of sulphur.
(a) What is the iron salt?
(b) Which type of reaction is this?
Answer:
(a) Ferrous sulphate
(b) Decomposition.
Question 29.
If you burn hydrogen in the presence of oxygen it will give water whereas if you electrolyse water it will give out hydrogen and oxygen.
(a) Which type of reaction takes place in first situation?
(b) Which type of reaction takes place in second situation?
Answer:
(a) Combination
(b) displacement.
Question 30.
What is formed when silver bromide is exposed to light?
Answer:
Silver metal and bromine vapour.
Question 31.
What is displacement reaction?
Answer:
The reaction in which a more reactive metal displaces the less reactive (or active) metal from its salt solution is called displacement reaction.
Question 32.
State chemical equation for;
(a) Iron reacting with steam,
(b) Magnesium reacting with dilute hydrochloric acid.
Answer:
(a) 3Fe + 4H2O → Fe3O4 + 4H2
(b) Mg + 2HCl → MgCl + H2
Question 33.
Look at the chemical equation.

(i) Identity ‘X’ and ‘Y’,
(ii) What type of reaction is this?
Answer:
(i) ‘X’ is Na2SO4 and ‘Y’ is BaSO4,
(ii) It is a double displacement as well as a precipitate reaction.
![]()
Question 34.
What is double displacement reaction?
Answer:
A chemical reaction in which two compounds react by an exchange of ions to form two new compounds is called double displacement reaction.
Question 35.
Look at reaction and state what is more reactive, ‘Mn’ or ‘Al’ and why.
3MnO2 + Al → 3Mn + 2Al2O3
Answer:
‘Al’ is more reactive than ‘Mn’ since ‘Al’ displaces ‘Mn’ from its oxide.
Question 36.
What is ion exchange?
Answer:
The process in which ions of one substance are replaced by similarly charged ions or another substance is called ion exchange.
Question 37.
Define oxidation reaction.
Answer:
The chemical reaction in which oxygen is added to a substance or hydrogen is removed from a substance is called oxidation.
Question 38.
What is reduction reaction?
Answer:
The reaction in which hydrogen is added to a substance or oxygen is removed from a1 substance is called reduction.
Question 39.
Why redox reaction Is called so?
Answer:
In redox reaction, oxidation as well as reduction occurs simultaneously. Hence, the reaction is called redox (reduction oxidation) reaction.
Question 40.
Give redox reaction when hydrogen suiphide reacts with chlorine.
Answer:
H2S+Cl2 → S+2HCl
![]()
Question 41.
Give the redox reaction when zinc oxide Is heated with carbon.
Answer:
ZnO+C → Zn+CO
Question 42.
What is oxidized and what is reduced in the following equation?
SO2 + 2H2S → 3S + 2H2O
Answer:
SO2 changes to S i.e; oxygen is reduced. H2S changes to S i.e. hydrogen is removed through oxidation.
Question 43.
State two common effects of oxidation in daily life.
Answer:
Oxidation causes
(a) Corrosion of metals and
(b) Rancidity in food.
Question 44.
What is corrosion?
Answer:
When a metal comes in contact of humid air, moisture or a chemical such as acid,the surface of metal starts getting eatenup.
This is called corrosion.
Question 45.
Give two examples of corrosion.
Answer:
(a) Silver ornaments turn black and
(b) Green coating gets deposited on copper vessel.
Question 46.
Give a chemical reaction showing rusting of iron.
Answer:
4Fe + 3O2 + 2xH2O → 2Fe2O3 x H2O
(Note: Here ‘x’ indicates the number of molecules of water and it keeps on varying.)
![]()
Question 47.
What is rancidity?
Answer:
The condition where in due to oxidation of fatty and oily foods such as snacks the food develops unpleasant smell and taste is called rancidity.
Fill in the Blanks
1. Formation of …………… is a proof as well as one of the indicators of chemical reaction.
Answer:
Precipitation
2. The characteristic of ………………. is observed to assure occurrence of chemical reaction when citric acid is added to potassium permanganate.
Answer:
Change in colour
3. Matter can neither be created, nor be destroyed. It is called the law of ………………
Answer:
conservation of mass.
4. On adding solution of substance ‘X’ to solution of ‘KI’, a yellow solid separates out from the solution. Now answer the two questions. (a) The substance ‘X’ is , (b) The solid yellow substance is ………………..
Answer: (a) Lead nitrate, (b) Lead iodide
5. Balancing in equation should be started from ………………
Answer:
The most complex substance in the reaction.
6. The reaction 2AgBr → 2Ag + Br2 is used in ……………
Answer:
Black and white photography.
7. ……………………. is formed when iron is heated with sulphur.
Answer:
Iron suiphide
8. Calcium oxide reacting vigorously with water to form slaked like is a type of ………………….. reaction.
Answer:
Combination
9. ………………….. is a more reactive metal among Fe and Mg.
Answer:
Mg
10. Formula for ferrous sulphate crystals is………………..
Answer:
FeSO2 . 7H2O
![]()
11. On passing electric current through water, ………………….. is/are obtained.
Answer:
Hydrogen and oxygen
12. Silver chloride when exposed to sunlight turns
Answer:
Grey
13. Hydrogen gas burns rapidly with sound.
Answer:
Popping
14. On submerging iron nail in copper sulphate solution the nail turns coloured.
Answer:
Reddish-brown
15. Generally, the double displacement reaction takes place in
Answer:
Solutions
16. Packets of chips are flushed with to prevent rancidity.
Answer:
Nitrogen
True Or False
1. ‘Respiration is an endothermic reaction’. — False.
2. ‘Adding potassium iodide to solution of lead nitrate gives green precipitate of lead iodide’. — False.
3. ‘Descriptive equation = Word equation’. — False
4. ‘It is mandatory to write physical states in chemical equation’. — False
5. ‘A decomposition reaction is similar to combination reaction’. — False
6. ‘Ferric oxide formed due to decomposition of ferrous sulphate is obtained in liquid state’. — False
7. During electrolysis of water, the volume of gas collected on negative electrode is same as the volume of gas collected on the positive electrode. — False
8. In reaction, \(\mathrm{MnO}_4^{2-} \rightarrow \mathrm{MnO}_2+\mathrm{MnO}_4^{1-}\mathrm{MnO}_4{ }^{2-}\) acts both as oxidizing agent as well as reducing agent. — True
9. In a double displacement reaction, one of the insoluble product precipitates. — True
10. The major cause of corrosion is hydrogenation. — False
11. Lime stone (CaCO3) decomposes on heating with evolution of CO2. — True
12. Calcium hydroxide combines slowly with oxygen present in air to form a white layer of calcium carbonate on the wall. — False
13. Among the following reactions only reaction number (ii) is a double displacement reaction.
(i) Pb + CuCl2 → PbCl2 + Cu
(ii) Na2SO4 + BaCl2 → BaSO4 + 2NaCl
(iii) C + O2 → CO2
(iv) CH4 + 2O2 → CO2 + 2H2O — True
![]()
14. Exposure of silver chloride to sunlight for a long duration turns it grey due to the formation of silver by decomposition of silver chloride. — True
15 The proportion by volume of H2 and O2 gases obtained t electrodes during electrolysis of water is 1 :1. — False
16. Precipitate obtained in precipitation reaction is soluble in water. — True
17. An element X when exposed to moist air turns reddish brown giving rise to a new compound Y. Here, X is Fe and Y is Fe2O3. — True
18. When lead (II) nitrate decomposes it produces lead (II) oxide, nitrogen dioxide and oxygen gas. In the balanced equation the value of co-officient of nitrogen dioxide is 4. — True
19. You cannot perform an oxidation reaction without a simultaneous reduction reaction. — True
Match the Following:
Question 1.
| A |
B |
|
1. Formation of iron sulphate and copper from iron and copper sulphate |
a. Decomposition reaction |
| 2. Formation of water from H2 and O2 |
b. Combination reaction |
| 3. Formation of ferric oxide, sulphur dioxode from ferrous sulphate |
c. Displacement reaction |
Answer: (1-c), (2-b), (3-a)
![]()
Question 2.
|
A |
B |
|
1. Copper sulphate |
a. Blue |
|
2. Ferrous sulphate |
b. Green |
|
3. Barium sulphate |
Answer: (1-a), (2-b)
Question 3.
| 1. Iron nail dipped in copper sulphate | a. Turns grey |
| 2. Silver chloride kept in China dish under sunlight | b. Turns brown |
| c. Turns faded blue |
Answer: (1-a) ,(2-b)