Haryana State Board HBSE 10th Class Science Important Questions Chapter 4 Carbon and Its Compounds Important Questions and Answers.
Haryana Board 10th Class Science Important Questions Chapter 4 Carbon and Its Compounds
Question 1.
Give a brief introduction of carbon.
Answer:
1. Carbon is a non-metallic element. Its symbol is C.
2. The amount of carbon in the atmosphere and earth’s crust is very less. Earth’s crust contain 0.02% carbon in the form of minerals such as carbonates, hydrogen carbonates, coal and petroleum. The atmosphere contains only 0.03% of carbon in the form of carbon dioxide.
3. In spite of being present in such a small quantity, carbon is an extremely important element. All the long things, plants and animals are made up of carbon based compounds known as organic compounds.
![]()
Atomic number and electron sharing:
1. The atomic number of carbon is 6. So, it has 2 electrons in Its first shell and 4 in the second or say outermost shell.
2. This makes the valency of carbon as 4 (i.e. 4 electrons in the outermost shell). This makes carbon quite a unique element. To complete the octet configuration, carbon either needs to lose 4 electrons or gain 4 electrons, both of which are not possible. Hence, carbon joins with other elements by sharing electrons and forming covalent bonds.
Question 2.
Explain electronic configuration, valency and bonding of carbon with other elements. OR ‘Carbon has a unique way of bonding’. Explain.
Answer:
1. The atomic number of carbon is 6. Hence, there are 2 electrons in its first (K) shell and 4 in second (L) i.e. outermost shell. Thus, carbon has 6 protons and 6 electrons.
2. The reactivity of an element is explained by its tendency to attain a completely filled outer shell to attain noble gas configuration.
3. Elements forming ionic compounds achieve noble gas configuration by either losing or gaining electrons from the outermost shell. The case of carbon is diUerent since it has 4 electrons in its outermost shell.
4. Carbon has to either gain or lose 4 electrons to attain noble gas configuration. The problem in doing this is discussed below:
- Carbon can gain 4 electrons to form C anion. If carbon does this, it will be difficult for the nucleus with 6 protons to hold 10 electrons (6 existing + 4 borrowed) Le. 4 extra electrons.
- Carbon can lose 4 electrons to form C4-’ cation. This would require a large amount of energy to remove 4 electrons leaving behind a carbon cation with 6 protons in its nucleus holding on to just 2 electrons.
Solution:
1. To overcome these problems, carbon neither accepts, nor gains but shares its valence electrons with other atoms of carbon or with atoms of other elements. The shared electrons belong to the outer shells of both the atoms. This way both the atoms attain noble gas configuration.
2. The bond formed by sharing of electrons in this manner is known as covalent bond.
3. Not only carbon but many other elements form molecules by sharing electrons and forming covalent bonds.
![]()
Question 3.
What do you mean by covalent bond and covalent compounds? Explain briefly with one example.
Answer:
Covalent bond and compounds :
1. Covalent compounds consist of molecules which are groups of atoms in which one or few pairs of atoms share the electrons by bonding.
2. In ionic bond, elements develop bonds by gaining or losing electrons but, in covalent bonds. elements develop bonds by sharing electrons.
3. For example, two atoms of hydrogen, each of them having one electron, share their electrons of the outermost orbit by forming a covalent bond and attain dual closed shell configuration of their nearby inert element, helium.
4. Here, both the hydrogen atoms jointly share the electrons for becoming inert and stable.
5. Only those electrons that are present in the outermost orbit of the atoms take part in bond formation.
6. The electron pair that takes part in sharing is known as bonding electron pair or bond electron pair.
Question 4.
What is an ionic bond and a covalent bond? Explain giving differences. OR State the key differences between an IonIc bond and a covalent bond.
Answer:
| Ionic bond (Electrovalent bond) |
Covalent bond |
| 1. The bond that takes place between a metal and a non-metal is called an ionic bond. 2. Bonding happens by ‘complete transfer of electrons’. Example: Bond between sodium (Na) metal and chlorine (Cl) non-metal to form NaCl, H2SO4, etc. |
1. The bond that takes place between two non-metals is called an covalent bond. 2. Bonding happens by mutual sharing of electrons’. Example: Bond between hydrogen (H) and hydrogen (H), Hydrochloric acid (HCl), methane (CH4), etc. |
![]()
Question 5.
Explain covalent bonding In hydrogen molecule. OR Explain covalent single bond with the help of an example.
Answer:
1. The atomic number of hydrogen is 1 and so hydrogen atom possesses one electron.
2. Hydrogen atom requires one more electron to achieve the closed shell configuration of near by inert element, helium.
3. Hence, two atoms of hydrogen, each having one electren, will share their one electron by forming a covalent bond, thus giving rise to hydrogen (H2) molecule.
4. Both these atoms will attain dual closed shell configuration like that of helium.
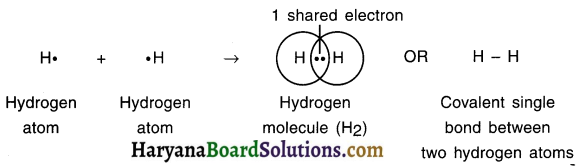
5. The electron pair that takes part in sharing is called bonding electron pair or bond electron pair.
6. The single line between two hydrogen atoms represent single covalent bond.
Question 6.
Explain covalent bonding in chlorine molecule.
Answer:
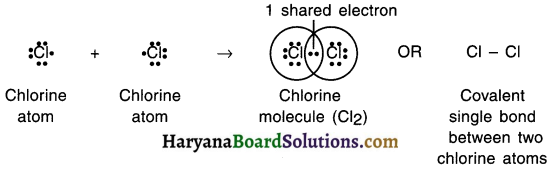
1. The atomic number of chlorine is 17 and so its electronic configuration is (2, 8, 7).
2. The chlorine atom will share the electron of its outermost orbit to attain the closed octet configuration of nearby inert gas.
3. Each chlorine atom requires one electron to attain octet configuration.
4. Hence, two chlorine atoms will share one-one electron with each other, form a single covalent bond, will attain octet configuration and form chlorine molecule.
Question 7.
Explain covalent bonding in oxygen. OR Explain covalent double bond with the help of an example.
Answer:
1. The atomic number of oxygen is 8 and so its electronic configuration is (2, 6).
2. Since the valency of oxygen is 2, each oxygen atom will share 2 electrons from its outermost orbit to complete the octet configuration.
3. Hence, two oxygen atoms will share two-two electrons with each other, form double covalent bond, will attain octet configuration and will form one oxygen (O2) molecule.
4. Because of the double covalent bond, this compound is called divalent compound.
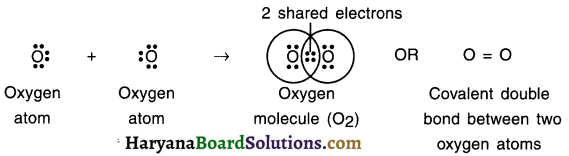
The double line between two oxygen atoms represent double covalent bond.
![]()
Question 8.
Explain covalent bonding in nitrogen. OR Explain covalent triple bond with the help of an example.
Answer:
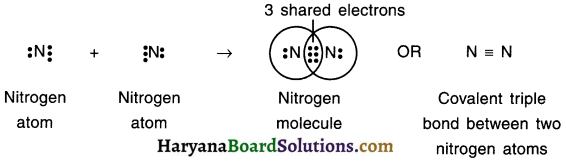
1. The atomic number of nitrogen is 7 and so its electronic configuration is (2, 5).
2. Since the valency of nitrogen is 3, each nitrogen atom will share 3 electrons from its outermost orbit to complete octet configuration.
3. Hence, two nitrogen atoms will share three-three electrons with each other, form triple covalent bond, attain octet configuration and form nitrogen molecule.
4. Because of the triple covalent bond, this compound is called trivalent compound.
Question 9.
Explain the formation of covalent bonds in water molecule (H2O).
Answer:
1. Oxygen is a central atom in the molecule of water. The atomic number of oxygen is 8,
so its electronic configuration is (2, 6)
2. Thus, oxygen has 6 electrons in its L shell and it needs two more electrons to fill the L shell.
3. The atomic number of hydrogen is 1 and so hydrogen atom possesses 1 electron in its K shell.
4. Oxygen shares two of its valence electrons with one electron each of K shell of two hydrogen atoms to form a molecule of water (H2O).
5. This way oxygen atom of water attains the electronic configuration of its nearest noble gas neon (Ne), while hydrogen atom attains the electronic configuration of its nearest noble gas helium (He) which has two electrons in its K shell.
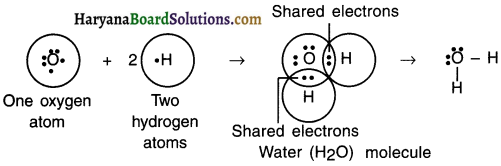
![]()
Question 10.
Explain covalent bonding in ammonia (NH3) molecule.
Answer:
1. Nitrogen is a central atom in the molecule of ammonia. The atomic number of nitrogen is 7, so its electronic configuration is (2, 5).
2. Nitrogen has five electrons in its L shell and so it needs three more electrons to fill the L shell.
3. Nitrogen shares three of its valence electrons with one electron each of K shell of three hydrogen atoms to form a molecule of ammonia (NH3).
4. Thus, nitrogen atom of ammonia attains the electronic configuration of its nearest noble gas neon (Ne), which has eight electrons in its L shell, while hydrogen atom attains the electronic configuration of its nearest noble gas helium (He), which has two electrons in its K shell.
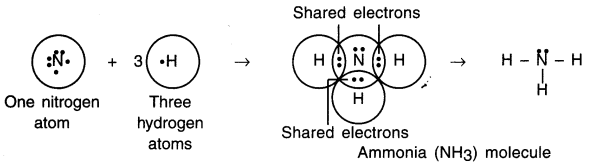
Question 11.
Explain covalent bonding in methane (CH4) molecule.
Answer:
1. Carbon is a central atom in the molecule of methane. The atomic number of carbon is 6, so its electronic configuration is (2, 5).
2. Thus, carbon has four electrons in its L shell and it needs four more electrons to fill the L shell.
3. Carbon shares its four valence electrons with one electron each of K shell of four hydrogen atoms to form a molecule of methane (CH4).
4. This way carbon atom of methane attains the electronic configuration of its nearest noble gas neon (Ne), which has eight electrons in its L shell, while hydrogen atom attains the electronic configuration of its nearest noble gas helium (He), which has two electrons in its K shell.
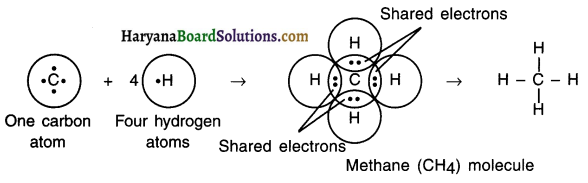
Question 12.
State the properties of covalent compounds (or carbon compounds).
Answer:
Properties of covalent compounds (or carbon compounds):
- Covalent compounds exists in all the three forms i.e. solid, liquid and gas.
- They have weak force of attraction between the molecules.
- They have lower melting and boiling points.
- They are non-conductors of electricity.
- Generally they are insoluble in water but soluble in organic solvents.
![]()
Question 13.
How does carbon bond with other atoms of carbon? OR Explain catenation.
Answer:
(i) Catenation:
- Carbon has a unique ability to bond with other atoms of carbon and form long chain. This unique
property of carbon is called catenation. - Catenation results in formation of large molecules. Moreover, the ability of carbon to bond with several elements results in formation of a large number of carbon based compounds.
(ii) Bonding by carbon:
- Carbon atom bonds with the help of three types of covalent bonds namely, single bond, double bond and triple bond.
(a) Single bond compound (Saturated compound):
- If carbon atom joins with another carbon atom with the help of only single bond then the compound formed is called a saturated compound.
(b) Double or triple bond (Un-saturated compound):
- If carbon atom joins with other carbon atoms via, double or triple bond, then the compounds formed are called unsaturated compounds.
- The carbon-carbon bond is very strong and hence stable. This gives rise to a large number of compounds with several carbon atoms linked with each other.
(iii) The structures formed by the three types of covalent bonds of carbon can be of the following types:
- Normal chain,
- Branched (Iso) chain or
- Cyclic chain
![]()
Question 14.
Why is carbon called tetravalent? How does It help carbon to bond?
Answer:
The valency of carbon is 4, ie. carbon has 4 electrons in its outermost shell. Hence, carbon is called tetravalent.
1. Since the valency of carbon is 4, it is capable of bonding with four other atoms of carbon or some mono-valent atoms i.e. atoms having one valency.
2. This way, carbon forms compounds with oxygen, hydrogen, nitrogen, sulphur, chlorine and many other elements and gives rise to several compounds.
Question 15.
State the two important properties of carbon that help it to form a large number of compounds.
Answer:
The two important properties of carbon that help it to form a large number of compounds are:
(1) Catenation:
- Carbon has a unique ability to bond with other atoms of carbon and form long chain. This unique property of carbon is called catenation.
- Catenation results in formation of large molecules. Moreover, the ability of carbon to bond with several elements results in formation of a large number of carbon based compounds.
- Carbon atom bonds with the help of three types of covalent bonds namely, single bond, double bond and triple bond.
(2) Tetravalency:
- The valency of carbon is 4, i.e. carbon has 4 electrons in its outermost shell. Hence, carbon is called tetravalent.
- Since the valency of carbon is 4, it is capable of bonding with four other atoms of carbon or some mono-valent atoms i.e. atoms having one valency.
- This way, carbon forms compounds with oxygen, hydrogen, nitrogen, sulphur, chlorine and many other elements and gives rise to several compounds.
(3) Other reasons:
- Carbon forms very strong bonds with other elements and so the compounds formed are extremely stable.
- No other element shows the property of catenation to the extent of carbon.
Question 16.
What are hydrocarbons? How are they classified?
Answer:
Hydrocarbons:
- Compounds containing hydrogen and carbon are called hydrocarbons.
- In organic chemistry, hydrocarbons are considered to be the simplest organic compounds.
Classification of hydrocarbons:
- Hydrocarbons are classified on the basis of the number of covalent bonds between carbon-carbon atoms.
- Thus, on the basis of covalent bonds, hydrocarbons can be classified as—
(I) Saturated hydrocarbons:
- Hydrocarbons having single covalent bonds between their carbon atoms are called saturated hydrocarbons.
- Alkanes are the main class of saturated hydrocarbons.
![]()
Question 17.
Give a classification of hydrocarbons. OR Differentiate between saturated and unsaturated hydrocarbons.
Answer:
Hydrocarbons can be classified into —
(i) Saturated hydrocarbons and
(ii) Unsaturated hydrocarbons
| Saturated hydrocarbons |
Unsaturated hydrocarbons |
| These hydrocarbons have single covalent bond between their carbon atoms. | These hydrocarbons have either double or triple covalent bonds between their carbon atoms. |
| Alkanes are the main class of saturated hydrocarbons. | Unsaturated hydrocarbons have two sub-types namely, (1) Alkenes and (2) Alkynes. |
| Alkanes have single covalent bonds between their carbon atoms. | Alkenes have double where as alkynes have triple covalent bonds between their carbon atoms. |
| Hydrocarbons under alkanes have suffix ‘ane’. | Hydrocarbons under alkenes have suffix ‘ene’ and those under alkynes have suffix ‘yne’. |
| Methane, propane, butane, etc. are alkanes. | Ethene, propene, butene, etc. are alkenes where as ethyne, propyne, butyne, etc. are alkynes. |
| General formula of alkanes is CnH2n+2 where n no. of carbon atoms. | General formula of alkenes is CnH2n and that of alkynes is CnH2n-2 where, n = no. of carbon atoms. |
Question 18.
Give the molecular, electronic and structural formula of methane
Answer:
1. Methane is the first member of the alkane series hydrocarbon.
2. Molecular formula, electronic formula and structural formula of methane are as under.
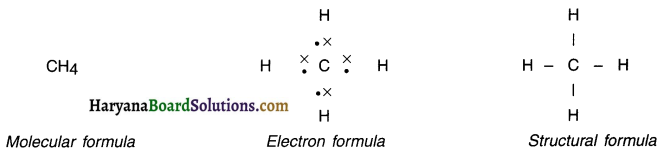
Question 19.
With the help of the example of ethane, show how to draw the structure of a hydrocarbon.
Answer:
1. Ethane is a hydrocarbon formed by joining carbon and hydrogen.
2. The molecular formula of ethane is C2H6.
![]()
Steps to draw the structure:
Step-1:
1. Since ethane is a saturated hydrocarbon, it will have a single covalent bond. First we link two carbon atoms by single bond.
2. Here, one valency of carbon is used. Now, three valencies are to be filled.
C – C
Step-2:
1. We now attach hydrogen atoms with carbon to satisfy remaining three valencies of carbon. This gives us the following structure.
2. Each carbon atom is bonded by a single covalent bond with carbon as well as hydrogen. So, all the valencies are filled and the structure is completed.
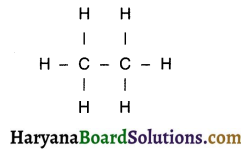
Question 20.
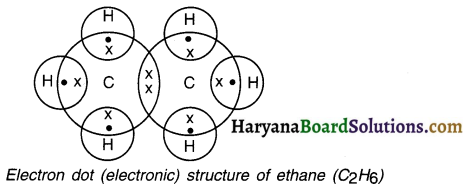
Draw the electron dot structure of ethane.
Answer:
Question 21.
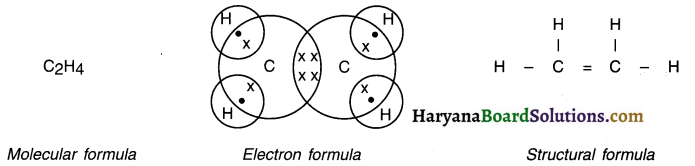
Give the molecular, electronic and structural formula of ethene.
Answer:
Molecular, electronic and structural formula of ethene are as under —
Question 22.
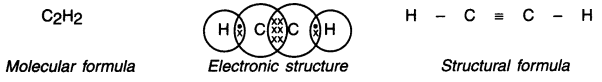
Give the molecular formula of ethyne and draw its electronic structure and bond structure.
Answer:
Ethyne (Acetylene):
1. Ethyne is the first member of the alkyne series.
2. Its molecular, electronic and structural formula are as follows —
![]()
Question 23.
Give formula and structure of first six alkanes
Answer:
Formula and structure of first six alkanes
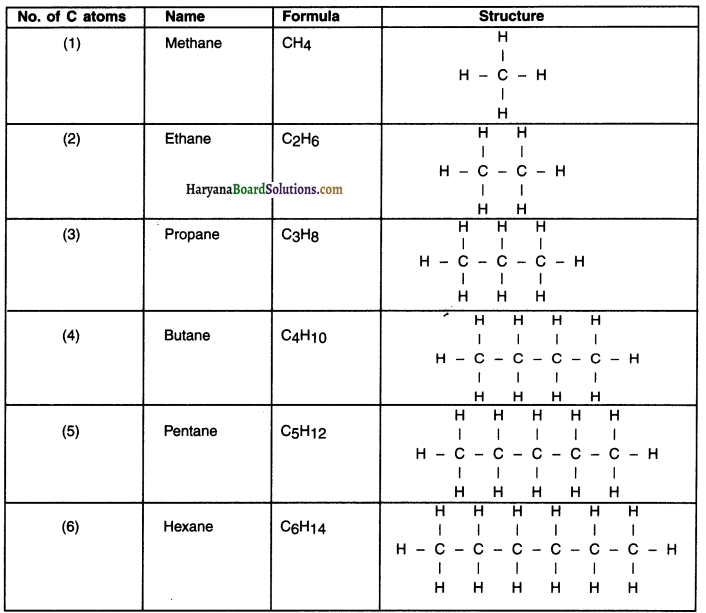
Question 24.
Explain Isomers and Isomerism.
Answer:
Isomers and Isomerism:
1. Property of catenation possessed by carbon gives rise to a large number of compounds with different structural formula and different physical properties.
2. Organic compounds that have same molecular formula but different structural formula are called isomers and the phenomenon is called isomerism.
3. ‘Iso’ indicates a branched chain whereas normal (-n) Indicates a straight chain structure.
4. As the number of carbon atoms increase in a chain, the number of isomers also increase.
5. For example, butane (C4H10) has only two isomers whereas hexane (C6-H14) has five isomers.
Example of ‘n’ i.e. normal and ‘iso’ i.e. branched structured hydrocarbons:
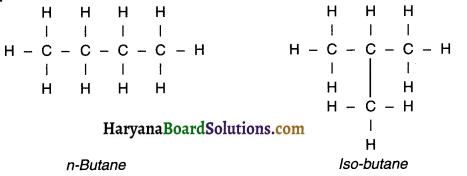
Question 25.
State the characteristics of isomers.
Answer:
Characteristics of isomers:
- Isomers have same molecular formula but different structural formula.
- Isomers have different melting and boiling points.
- They have different chemical properties.
![]()
Question 26.
Explain the classification of hydrocarbon compounds based on their different structures.
Answer:
On the basis of their structures (arrangement of carbon atoms), hydrocarbon compounds can be classified into following three categories:
(a) Straight chain compounds,
(b) Branched chain compounds
(c) Ring structures OR Cyclic compounds
(a) Straight chain compounds:
Hydrocarbon compounds in which all carbon atoms are arranged linearly i.e. in a straight chain are called straight chain compounds.
For example, propane (C3H8):
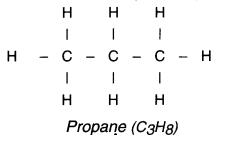
Branched chain compounds:
A hydrocarbon compound in which carbon atoms are arranged in straight chain, as well as possess one or more branches is called a branched chain compound.
For example, Carbon-skeleton of four carbon atoms of butane can be arranged in two different possible ways as follows:
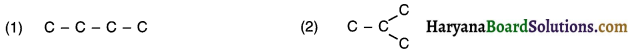
Now, when we satisfy the remaining valencies of carbon with hydrogen we get the following structures:
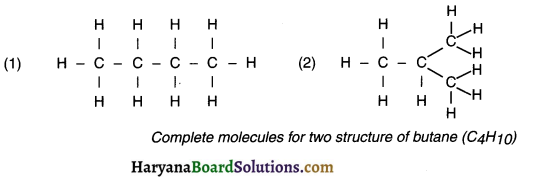
(c) Ring structure OR Cyclic compounds:
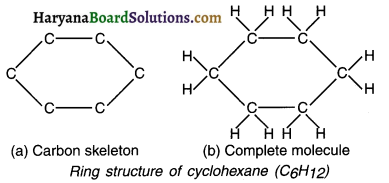
Hydrocarbon compounds in which the first carbon atom is directly linked with the end (last) carbon atom, forming ring or cyclic structures are called ring structures or cyclic compounds. For example, cyclohexane (C6H12).
Question 27.
Is cyclohexane an isomer of hexane? Explain with reason.
Answer:
1. No. The molecular formula of hexane is C6H14 whereas that of cyclohexane is C16H12.
2. Since the molecular formula of cyclohexane is different than hexane it, is not the isomer of hexane.
Question 28.
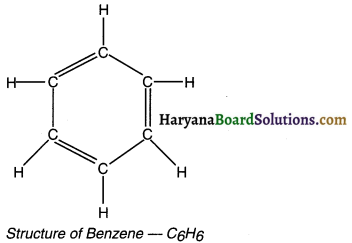
Draw the structure of benzene.
Answer:
The molecular formula of benzene is C6H6.
Question 29.
What are cyclic hydrocarbons? Draw structure of two cyclic hydrocarbons.
Answer:
Ring structure OR Cyclic compounds:
Hydrocarbon compounds in which the first carbon atom is directly linked with the end (last) carbon atom, forming ring or cyclic structures are called ring structures or cyclic compounds. For example, cyclohexane (C6H12).

The molecular formula of benzene is C6H6.

Question 30.
What are heteroatoms? Explain.
Answer:
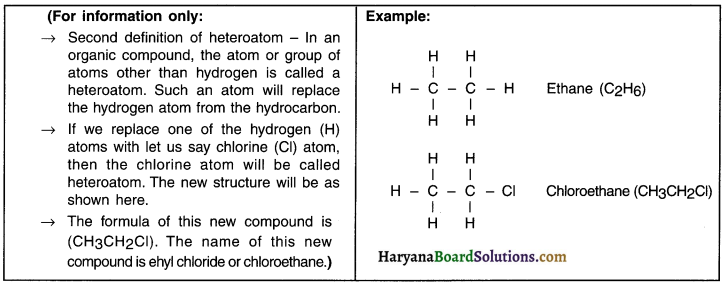
Heteroatom:
1. Apart from hydrogen, carbon forms bonds with other elements as well.
2. The atom or group of atoms of an element that replaces hydrogen in a hydrocarbon is called an heteroatom.
3. Oxygen (O), nitrogen (N), sulphur (S), halogens such as fluorine (F), chlorine (Cl), bromine (Br) and iodine (I)) are typical heteroatoms that replace hydrogen.
![]()
Question 31.
What are functional groups? Explain.
Answer:
Functional groups:
1. A heteroatom (i.e. an atom or a group of atoms) which imparts specific properties to the organic compound they are attached to is called a functional group. (Note: When an heteroatom attaches to an organic compound, the physical and chemical properties of that compound changes. The heteroatom which Is responsible for changes in these properties Is called functional group.)
2. Thus, the functional group decides the physical and chemical properties of the carbon compound, irrespective of the length of the carbon chain.
3. There are several functional groups. Some of them are listed in the table below.
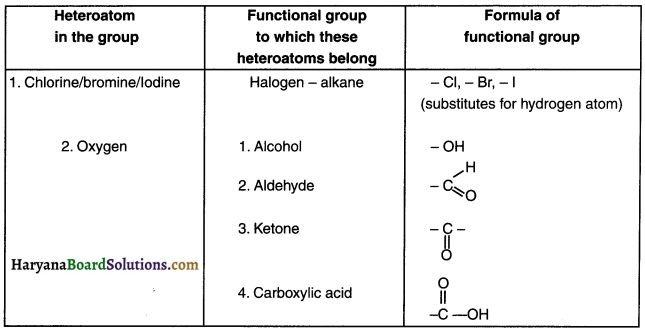
Question 32.
Explain how does the name of an organic compound changes when a functional got is attached to it
Answer:
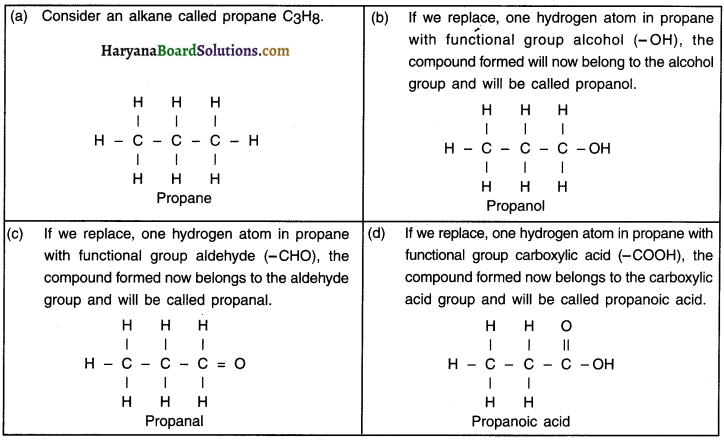
Question 33.
“The number of carbon atoms in ethane, ethanol and ethanoic acid are same, yet all the three compounds show different physical and chemical properties”. Explain.
Answer:
1. Ethane is an alkane and its formula is C2H6.
2. When functional group hydroxyl – OH attach to ethane, the compound formed is ethanol or ethyl alcohol and its formula is C2H5OH. Similarly, when functional group carboxylic acid – COOH attach to ethane, the compound formed is ethanoic acid which is an acid and its formula is CH3COOH.
3. In a hydrocarbon, the functional group changes the physical and chemical properties of the compound.
4. Now, although the number of carbon atoms in all the three compounds i.e. ethane, ethanol and ethanoic acid are same, yet all the three compounds show different physical and chemical properties because ethane has no functional group, ethanol contains functional group alcohol whereas ethanoic acid consists of another functional group called carboxylic acid.
![]()
Question 34.
What do you mean by homologous series? Give its characteristics.
Answer:
Homologous series:
1. The series of organic compounds in which a particular functional group attaches to the carbon chain in place of hydrogen atom is called a homologous series.
2. Each compound of the homologous series differs from its previous or later compound by (CH2).
3. For example, the alkanes namely methane (CH4), ethane (C2H6), propane (C3H8) and so on form a homologous series with a definite difference of CH2.
4. Similarly, CH3OH, C2H5OH, C3H7OH is the homologous series of alcohols with functional group – OH and with a definite difference of CH2 between each compound.
Question 35.
State the characteristics of homologous series.
Answer:
Characteristics of homologous series:
1. Each member of the homologous series contains same elements as well as same functional group.
2. Each member of the homologous series can be expressed by a general formula. For example, general formula of alkane series of hydrocarbc,s is CnH2n+2.
3. The difference in molecular formula between two successive members of the series of homologous compounds is CH2.
4. Same prefix or suffix is applied to the nomenclature of each member of the series. For e.g., suffix sane’ is put to each member of the alkane series.
5. Difference between molecular masses of any two successive members of the series is 14u.
6. As the number of carbon and hydrogen increases in the series, the molecular mass of the compound also increases.
- As the molecular mass of the compound increases, the physical properties such as boiling point, melting point, solubility, etc. also change gradually.
7. The chemical properties of each compound of a homologous series remain same.
Question 36.
What is IUPAC name?
Answer:
IUPAC names:
1. Organic compounds or hydrocarbons have two names, (a) Common name and (b) IUPAC name.
2. IUPAC names are names given by IUPAC (Note: IUPAC = International Union of Pure and Applied Chemistry. These names are also called JUPAC nomenclature.)
Example: Methyl alcohol is an alcohol formed from methane. Methyl alcohol is a general (common) name where as methanol is its IUPAC name.
Question 37.
State the method to name the carbon compounds (hydrocarbons). OR Explain nomenclature of organic compounds.
Answer:
Nomenclature of organic compounds:
(Note: The number of carbon atoms in a hydrocarbon (or any other organic compound) is indicated by using following steps. The table is only for understanding purpose. Actual answer starts after the table.)
|
No. of carbon atoms |
Representation |
| 1 — Carbon atom is indicated by writing: 2 — Carbon atoms are indicated by writing: 3 — Carbon atoms are indicated by writing: 4 — Carbon atoms are indicated by writing: 5 — Carbon atoms are indicated by writing: |
‘Meth’ |
Steps for naming:
The names of compounds in homologous series are based on the name of the basic carbon chain which are modified by either a ‘prefix’ or a ‘suffix’ of the functional group.
(i) Identify the ‘number of carbon atoms’ in the compound. (Note: Refer the note above. Based on it, lets say a carbon compound has 3 carbon atoms, then its prefix would be ‘prop’.)
- If the compound is a saturated hydrocarbon with 3 carbon atoms and without any functional group, it will belong to alkane series and its name would be prop + ane = propane.
(ii) If there is a functional group present, it will be indicated with either a prefix or a suffix. For example, C3H7OH has 3 carbon atoms and has a functional group ‘-OH’ i.e. alcohol. So name of the compound is propanol.
(iii) As discussed in point (ii) if the name of the functional group is to be given as suffix, the name of the carbon chain is modified by replacing the last letter ‘e’ with proper suffix. For example, a 3-carbon chain with a ketone functional group would be named in the following manner:
Propane = Propan + ‘one’ = Propanone.
![]()
(iv) If the carbon chain is unsaturated (i.e. having double or triple covalent bond), the final ‘ane’ in the name of carbon chain is replaced by ‘ene’ or ‘yne’.
For example, a 3-carbon chain with a propene bond would be called propene and if it has a triple bond, it will be called propyne.
Nomenclature of Organic Compounds based on Functional Groups:
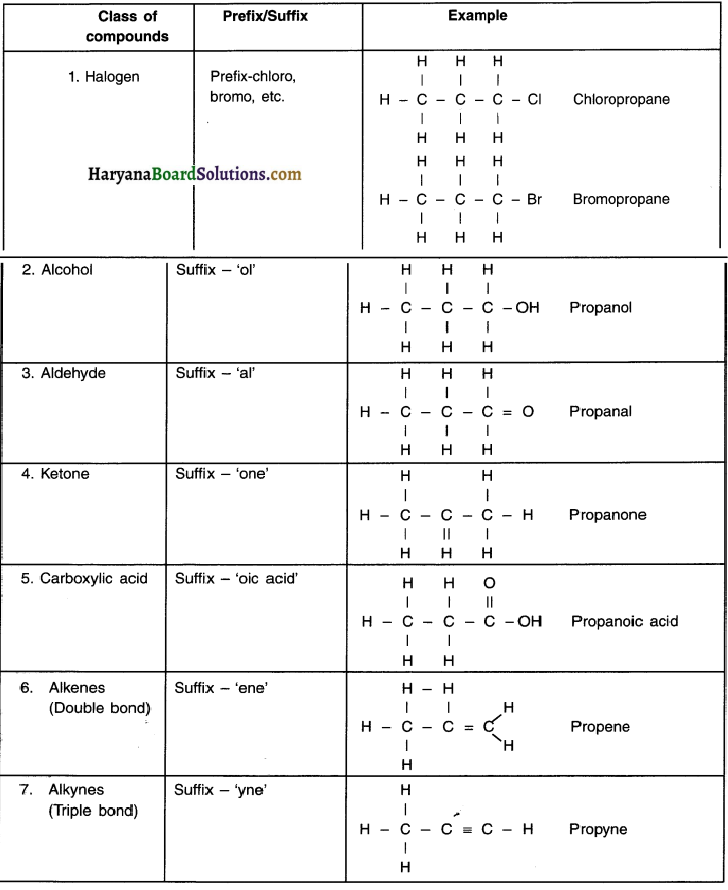
Question 38.
Discuss chemical properties of carbon compounds briefly.
(Note: Each property can be asked as a separate question.)
Answer:
Chemical properties of carbon compounds:
(i) Combustion:
Carbon present in all its allotropes burn in sufficient amount of oxygen. On burning, it produces carbondioxide and water and liberate heat and light. These reaction are oxidation reactions.
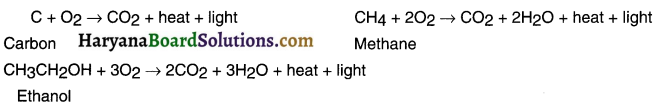
(ii) Oxidation:
- Oxidation is the reaction in which carbon compounds take up oxygen in the presence of oxidizing agents to give another compound.
- There are certain substances which are capable of adding oxygen to others i.e. adding oxygen to reactants. Such materials are called oxidizing agents.

Example:
Ethanol (ethyl alcohol) gets oxidized into ethanoic acid (i.e. a carboxylic acid) in the presence of oxidizing agent alkaline potassium permanganate or acidified potassium dichromate.
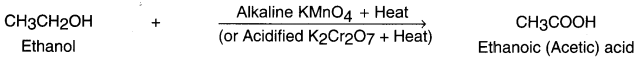
(iii) Addition (Hydrogenation) reaction:
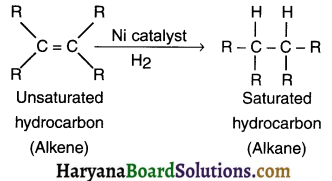
- A reaction in which adding one molecule to an organic compound gives a new but single organic compound is called addition reaction.
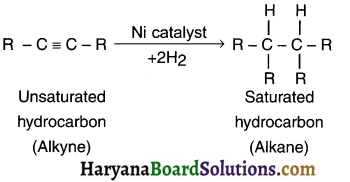
- For example, on adding hydrogen to an unsaturated (alkene or alkyne) hydrocarbon in the presence of catalyst such as palladium or nickel gives a single but saturated (alkane) product. This reaction is called addition reaction.
(1) Addition reaction In alkenes:
(2) Addition reaction in alkynes:
(iv) Substitution reaction:
- A reaction in which one or more hydrogen atoms of a hydrocarbon are substituted (replaced) by some other atom(s) (such as chlorine) is called a substitution reaction.
- Actually saturated hydrocarbons are quite unreactive and remain inert in the presence of most reagents. However, in the presence of sunlight, these compounds ie. alkanes undergo substitution reaction.
Example:
Methane (an alkane) reacts with chlorine in the presence of sunlight to form chloromethane and hydrochloric acid. In this reaction, one hydrogen (H) atom of methane gets substituted by a chlorine (Cl) atom. This converts CH4 into CH3Cl.
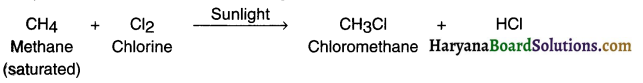
The way chlorine replaced one hydrogen atom from methane, it rapidly replaces each hydrogen atom with chlorine atom one by one.
Question 39
Give an example of addition reaction along with necessary chemical reaction.
Answer:
When hydrogen is added to ethene (an unsaturated hydrocarbon) and heated in the presence of nickel catalyst it gives a single saturated product ethane.
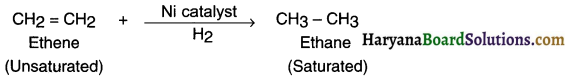
Question 40.
State the non difference between combustion and oxidation. OR ‘All combustion reactions are also oxidation reactions but, the reverse is not true.
Answer:
1. During combustion, a carbon compound is burnt in the air to give out carbon dioxide and water and liberate heat and light.
2. During oxidation the carbon compounds take up oxygen in the presence of oxidizing agents to give another carbon compound. Hence, all combustion reactions are also oxidation reactions but, the reverse is not true.
![]()
Question 41.
What is hydrogenation of oils? OR How ¡s vegetable ghee prepared from vegetable oil? OR Explain addition reaction giving example of vegetable oil.
(Note: Hydrogenetaion = Addition of hydrogen)
Answer:
1. Vegetable oils have long unsaturated fats having double bonds between sorne of their carbon atoms.
2. When a vegetable oil (like groundnut oil) is heated with hydrogen gas in the presence of nickel catalyst, the oil turns into a saturated fat called vegetable ghee or say naspati ghee. This reaction is known as hydrogenation of oils.
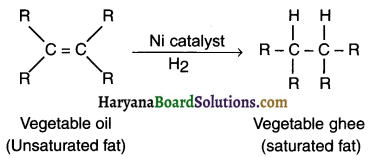
Vegetable oil is in liquid form which after hydrogenation turns into semi-solid ghee.
Question 42.
What is ethanol? State its properties.
Answer:
Ethanol: Ethanol is the second member of the homologous series of alcohol. Its formula is C2H2OH.
Ethanol or ethyl alcohol is the most common and most widely used alcohol and hence is also simply called alcohol.
Properties:
- At room temperature it exists in the liquid form.
- Ethanol is a very good solvent. It is soluble in water in any proportion.
Question 43.
State the uses and abuses of ethanol.
Answer:
Uses of ethanol:
- Ethanol is the active ingredient of all alcoholic drinks.
- Ethanol is a good solvent and so it is also used to make medicines such as tincture iodine, cough syrups and several other tonics.
Abuses:
- Consuming pure (undiluted) ethanol even in a small quantity can prove lethal.
- Consuming diluted ethanol that too in small quantity causes drunkenness.
- People fall prey and addicted to alcoholic dnnks. This ruins individual health, family and society at large.
![]()
Question 44.
State reactions (chemical properties) of ethanol.
Answer:
Reactions of ethanol:
(i) Reactions with sodium:
Ethanol reacts with sodium and produces sodium ethoxide along with evolution of hydrogen gas.

(ii) Dehydration:
- When ethanol is heated with excess concentrated sulphuric acid at 443 K, it gets dehydrated to form ethene.
- The concentrated sulphuric agent works as a dehydrating agent which removes water from ethanol.

Question 45.
What is ethanoic acid? State its physical properties.
Answer:
Ethanoic acid:
- Ethanoic acid is the second member of the homologous series of carboxylic acids.
- Its formula is CH3COOH. The common name of ethanoic acid is acetic acid.
Properties of ethanoic acid:
- Ethanoic acid is a weak acid.
- It is sour in taste. 5-8% solution of ethanoic (acetic) acid in water is called vinegar. Vinegar is widely used as preservative in pickles and in preparation of certain food items.
- The melting point of pure ethanoic acid is 290 K i.e. just nearly 17° Celsius. As a result, it often freezes in winter.
- The frozen form of ethanoic acid looks like solid ice or say ‘glacier’. Hence, it is also called ‘glacial acetic (or ethanoic) acid’
Question 46.
Discuss the chemical properties (reactions) of ethanoic acid.
Answer:
Chemical properties (reactions) of ethanoic acid:
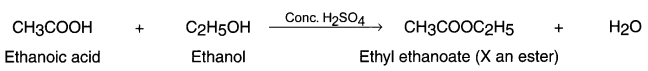
(i) Esterification reaction:
- When acid reacts with alcohols in the presence of little amount of concentrated sulphuric acid, the reaction produces esters. This reaction is called esterification reaction.
- Ester is a sweet smelling substance.
- When ethanoic acid reacts with ethanol in the presence of concentrated sulphuric acid, ester is produced.
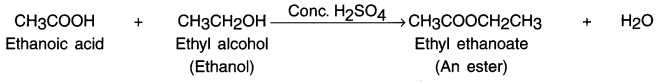
(ii) Reaction with base Le. alkali (Saponification reaction):
When the ester formed in above reaction is heated with sodium hydroxide (a base) solution then the ester breaks down to give back original alcohol Le. ethanol and sodium salt of the carboxylic acid.
This reaction is called saponification because ills used in making soap.
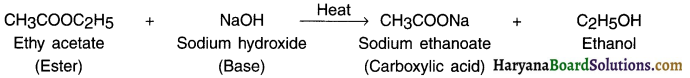
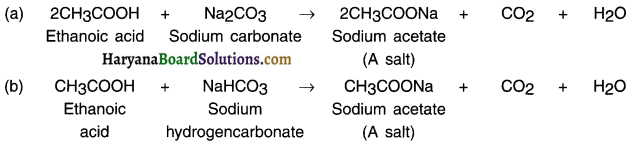
(iii) Reaction with carbonates and hydrogencarbonates:
Ethanoic acid reacts with carbonates and hydrogen carbonates to produce salt, carbon dioxide and water. The salt produced is commonly called sodium acetate.
Question 47.
What is esterification?
Answer:
Esterification reaction:
- When acid reacts with alcohols in the presence of little amount of concentrated sulphuric acid, the reaction produces esters. This reaction is called esterification reaction.
- Ester is a sweet smelling substance.
- When ethanoic acid reacts with ethanol in the presence of concentrated sulphuric acid, ester is produced.
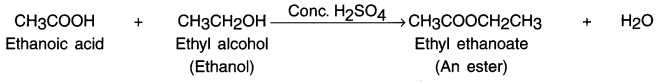
Question 48.
What Is an ester? State Its properties.
Answer:
1. Ester is a sweet smelling substance.
2. It is used in making perfumes and as a flavouring agent.
3. On treating ester with sodium hydroxide (a base alkali), the ester converts back to alcohol and sodium salt of carboxylic acid. This reaction is called the saponification process.
![]()
Question 49.
How does ethanoic acid react with alkali? OR What is saponification? State its reaction.
Answer:
Reaction with base Le. alkali (Saponification reaction):
When the ester formed in above reaction is heated with sodium hydroxide (a base) solution then the ester breaks down to give back original alcohol Le. ethanol and sodium salt of the carboxylic acid.
This reaction is called saponification because ills used in making soap.

Question 50.
State and explain chemical reaction of ethanoic acid with carbonates and hydrogencarbonates.
Answer:
Reaction with carbonates and hydrogencarbonates:
Ethanoic acid reacts with carbonates and hydrogen carbonates to produce salt, carbon dioxide and water. The salt produced is commonly called sodium acetate.

Question 51.
What is soap? Draw and explain the structure of its molecule.
Answer:
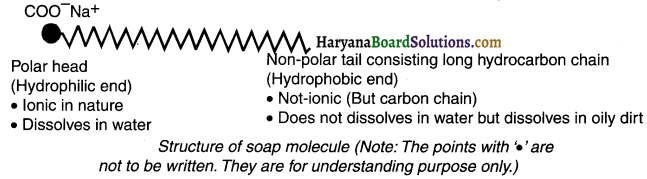
Soap:
1. A molecule of soap is a sodium or potassium salt of long chain carboxylic acid.
2. Each long chain soap molecule is made up of two parts. They are —
- a polar head and
- a polar tail.
The polar head (Hydrophilic end): It is made up of functional group sodium carboxylate ( – COONa). It is ionic ¡n nature. Moreover, it is hydrophilic which means it attracts water (or say dissolves in water).
The non-polar tail (Hydrophobic end): It is a long hydrocarbon chain. It is not ionic. It is hydrophobic which means it repels water (but dissolves in oil) or say dirt.
Question 52.
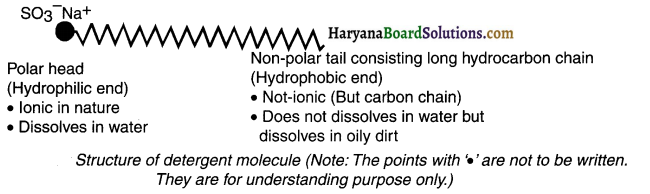
What are detergents? Explain their molecular structure.
Answer:
Detergents:
- Detergent is a chemical substance used for cleaning purposes.
- A molecule of detergent is ammonium or sulphonate salt of long chain carboxylic acid.
- In detergent, the functional group sodium sulphonate (-SO3Na) is attached to the long chain of hydrocarbon.
![]()
Question 53.
How does a soap cleans the dirt from clothes? Explain.
Answer:
Cleansing action of soap:
1. When soap is added in water that has dirty clothes —
- The polar head (hydrophilic or ionic end) of the soap molecule dissolves in water whereas the
- The non-polar tail (hydrophobic end) dissolves in oily dirt.
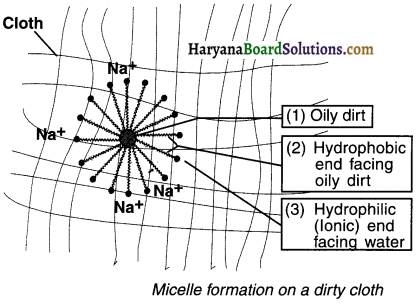
2. The soap molecule dissolves and get arranged in a systematic orientation where in the head portion faces towards the water and the tail faces the dirt. Due to this orientation, the oily dirt will get trapped at the centre of the micelle. Such patterns having ring like structures are called micelles and the process is known as micelle formation.
3. The micelles capture the dirt particles within their rings and suspend them in water. Finally, when we brush and rub the clothes, the dirt gets removed from the clothes.
Question 54.
How are micelles formed?
Answer:
1. When soap is added in water that has dirty clothes —
- The polar head (hydrophilic or ionic end) of the soap molecule dissolves in water whereas the
- The non-polar tail (hydrophobic end) dissolves in oily dirt.
2. The soap molecule dissolves and get arranged in a systematic orientation where in the head portion faces towards the water and the tail faces the dirt. Due to this orientation, the oily dirt will get trapped at the centre of the micelle. Such patterns having ring like structures are called micelles and the process is known as micelle formation.
Question 55.
What is the advantage of detergent over soap? OR Use of detergent has increased compared to washing soap. Give reason. OR The limitations of soap ¡s overcome by a detergent. Explain.
Answer:
1. Hard water contains calcium (Ca) and magnesium (Mg) salts.
2. When hard water is used during washing, the salts of Ca and Mg react with soap to form insoluble salts or say precipitates of Ca and Mg. Also called scum. The scum does not dissolve in water. Hence, more soap is used for cleaning.
3. On the other hand, detergent reacts with Ca and Mg salts of hard water and forms soluble salts of Ca and Mg. These salts remain in water and so very less amount of detergent is needed for washing.
4. Hence, the use of detergent has increased compared to washing soap.
Question 56.
Differentiate between soap and detergent.
Answer:
|
Soap |
Detergent |
| Soaps are sodium salts of long chain of carboxylic (fatty) acids. | Detergents are sodium salts of long chain of suIphonates. |
| The functional group in soap is COONa. | The functional group in detergent is -SO3Na. |
| Soap forms insoluble precipitates with calcium and magnesium present in hard water. | Detergent does not form insoluble precipitates with calcium and magnesium present in hard water. |
| Soap is not suitable for washing purposes when the water is hard. | Detergent is suitable for washing purposes even if the water is hard. |
| Cleansing effect of soap is not as good as detergent. | Cleansing effect of detergent is better than soap. |
Question 57.
Functional groups play a key role in organic compounds. Give reason.
Answer:
1. An atom or a group of atoms responsible for chemical behaviour of the parent molecule is called a functional group.
2. Different molecules that contain same kind of functional group or groups undergo similar reactions.
3. By learning the properties of these functional groups, we can study and understand properties of many organic compounds.
4. As a result, functional groups play a key role in organic compounds.
Question 58.
In a homologous series, as you progress in the series, the physical properties change but chemical properties do not. Give reason.
Answer:
1. In a homologous series, as the series progresses, the number of carbon and hydrogen atoms increase. This means the molecular mass of every next compound in the series is more than the previous compound.
2. Physical properties such as boiling point, melting point, density, solubility, etc. are dependent on the molecular mass. Since, the molecular mass gradually increase in the series, the compounds show change in physical properties.
3. The chemical properties of any homologous series are determined by the functional group of the series. Since, all the compounds of a given series have the same functional group, the chemical properties of all the compounds remain same.
Question 59.
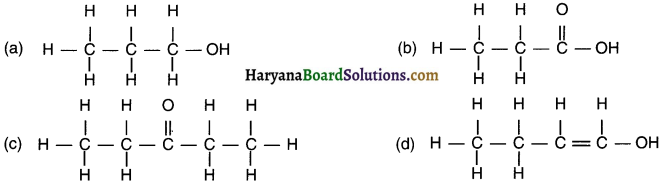
Write the names of the following compounds.
Answer:
(a) This compound contains five carbon C atoms and – COOH functional group. Hence, its name is Pentanoic acid.
Derivation of name: Remove ‘e’ from Pentane and add ‘oic acid’ for functional group – COOH = Pentanoic acid.
(b) This corn pound contains five carbon C atoms along with one triple bond. Hence, its name is Pentyne.
Derivation of name: Remove ‘e’ from Pentane and add ‘yne’ = Pentyne.
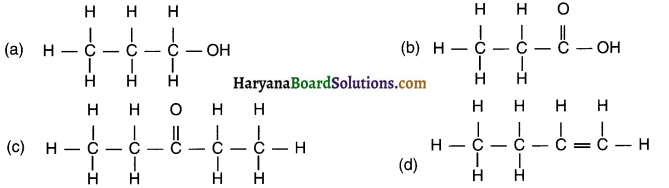
(c) This compound contains seven carbon C atoms and – CHO functional group. Hence, its name is Heptanal.
Derivation of name: Remove ‘e’ from Heptane and add ‘al’ for functional group – CHO = Heptanal.
(d) This compound contains five carbon C atoms and – OH functional group. Hence, its name is Pentanol.
Derivation of name: Remove ‘e’ from Pentane and add ‘ol’ for functional group – OH = Pentanol.
Question 60.
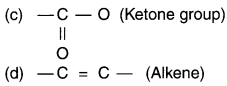
Identify and name the functional groups present in the following compounds.
Answer:
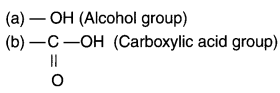
Question 61.
How can we differentiate between saturated and unsaturated hydrocarbons on the basis of combustion?
Answer:
1. Saturated hydrocarbons burn with a blue flame i.e. a clean flame which neither emits smoke nor leaves sooty deposit.
2. In unsaturated hydrocarbons, the percentage of carbon is higher. Hence, they burn in air producing a yellow sooty flame.
3. Thus, by studying the type of flame and the residue we can differentiate between saturated and unsaturated hydrocarbons.
![]()
Question 62.
Why kerosene stove burns with a blue flame but a kerosene lantern with a yellow?
Answer:
1. The construction of stove is such that it allows oxygen-rich air to enter into the stove in sufficient quantity. So, the stove burns with a blue flame.
2. The lantern is covered with glass to prevent the flame from getting extinguished. Hence, there is limited supply of oxygen available to lantern and so it burns with a yellow flame.
Question 63.
A compound X ¡s formed by the reaction of a carboxylic acid C2H4O2 and an alcohol in presence of a few drops of H2SO4. The alcohol on oxidation with alkaline KMnO4 followed by acidification gives the same carboxylic acid as used in this reaction. Give the names and structures of (a) carboxylic acid, (b) alcohol and (c) the compound X. Also write the reaction.
Answer:
(a) Carboxyllc acid having molecular formula C2H4O2 is acetic acid (or ethanoic acid) Its structure is
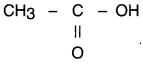
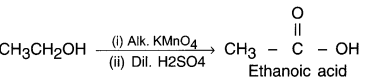
(b) Since, an alcohol which on oxidation with alkaline KMnO4 followed by acidification gives ethanoic acid. it must be ethanol. Its structure is CH3CH2 – OH.
Question 64.
Which oil and ghee are good for health and which should be avoided?
Answer:
1. Vegetable oils containing unsaturated fat are good for health and hence should be used for cooking. Sun-flower oil, groundnut oil, etc. are some of them.
2. Vegetable ghee contains saturated fat and hence is not good for health.
3. Animal fats found in butter and desi ghee also contain saturated fats. But, they can be eaten in moderate quantity to maintain good health.
Question 65.
What happens when hydrogen atoms of methane are one by one replaced with chlorine atoms in the presence of sunlight?
Answer:
1. Formula of methane is CH4.
2. When one hydrogen is replaced by chlorine, we get chioromethane (CH3Cl), when two hydrogen are replaced by two chlorine atoms we get dichioromethane (CH2Cl2), when three hydrogen atoms are replaced by three chlorine atoms we get chloroform or carbon trichloride (C3Cl4). Finally, when four hydrogen atoms are replaced by four chlorine atoms, we get carbon tetrachioride (CCl4).
Question 66.
Consumption of alcohol should be avoided. Give reason.
Answer:
1. Consuming pure (undiluted) ethanol e. alcohol even in a small quantity can prove lethal.
2. Consuming diluted ethanol that too in small quantity causes drunkenness.
3. People fall prey and addicted to alcoholic drinks, This wins individual health, family and society at large.
4. Due to all these harmful effects, it is said that consumption of alcohol should be avoided.
Question 67.
Ethene is formed when ethanol at 443 K is heated with excess of concentrated sulphuric acid. What is the role of sulphuric acid in this reaction? Write the balanced chemical equation of this reaction.
Answer:
When ethanol is heated with excess of concentrated sulphuric acid at 443 K, it gets dehydrated to form ethene.

Here, concentrated sulphuric acid acts as a dehydrating agent which removes water molecule from the ethanol molecule.
Question 68.
What is the role of metal or reagents written on arrows in the given chemical reactions?
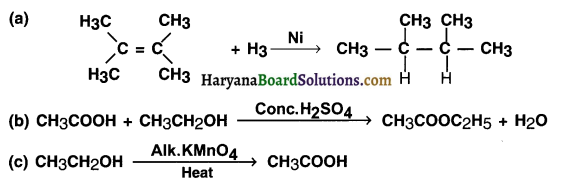
Answer:
(a) Nickel (Ni) acts as the catalyst during the reaction.
(b) Conc. H2SO4 increases the rate of the forward reaction. In other words, conc. H2SO4 acts as a catalyst which works as a dehydrating agent.
(c) Alkaline KMnO4 acts as an oxidizing agent and oxidizes ethanol to ethanoic acid.
![]()
Question 69.
Explain the given reactions with the examples.
(a) Hydrogenation reaction
(b) Oxidation reaction
(c) Substitution reaction
(d) Saponification reaction
(e) Combustion reaction
Answer:
(a) Hydrogen reaction:
Addition of hydrogen to the unsaturated molecule for making it saturated is known as hydrogenation.
Example:
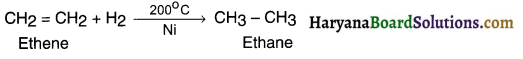
(b) Oxidation reaction:
The reactions in which an oxidizing agent supplies nascent oxygen for oxidation are called oxidation reactions.
![]()
(c) Substitution reaction:
When one atom or a group of atoms replaces or substitutes another atom or a group of atoms from the molecule, It is known as substitution reaction.
![]()
(d) Saponification reaction:
When esters are hydrolyzed in the presence of a base (NaOH) then the reaction is called saponification reaction.
Example: CH3COOCH3 + NaOH → CH3COONa + CH3OH
(e) Combustion reaction:
Organic compounds burn readily in air to form CO2 and water vapour along with lot of heat. Such a reaction is known as combustion reaction. Example: C2H5OH + 3O2 → 2CO2 + 3H2O + Energy
Very Short Answer Type Question :
Question 1.
State the atomic number of carbon and its electronic configuration.
Answer:
Atomic number Z = 6. Electronic configuration: K – 2, L – 4.
Question 2.
What defines the reactivity of an element?
Answer:
The tendency to lose or gain the electrons so as to complete the outer shell to attain noble gas configuration decides how reactive an element would be.
Question 3.
How does carbon bonds?
Answer:
Carbon shares its valence electrons with other carbon atoms or with atoms of other elements for joining purpose and attaining noble gas configuration. The bond formed while sharing is covalent bond.
Question 4.
What is a covalent bond?
Answer:
A chemical bond formed between two or more atoms by mutual sharing of valence electrons is known as a covalent bond.
Question 5.
Why is carbon called tetravalent?
Answer:
Since the valency of carbon is 4, it is called tetravalent (tetra = 4, valent = valency)
Question 6.
Which two important characteristics of carbon are responsible for formation of a very large number of carbon compounds?
Answer:
Catenation and tetravalency
Question 7.
In which forms is carbon available?
Answer:
In free state carbon occurs as diamond and graphite. In combined state, carbon occurs in the form of compounds such as CO2 in the air, carbonates, fossil fuels, etc.
Question 8.
Why covalent compounds have low melting and boiling point?
Answer:
Covalent bonds of compounds are weaker as compared to ionic bonds. Hence, in general, covalent compounds have lower melting and boiling points.
![]()
Question 9.
Why covalent compounds are bad conductors of electricity?
Answer:
Compounds having covalent bonds do not have ions or free electrons that conduct electricity. Hence, covalent compounds do not form strong electrolytes and so they are not very good conductors of electricity.
Question 10.
What is the problem with silicon exhibiting catenation property?
Answer:
Silicon can show catenation property by forming compounds with hydrogen which have chains upto seven or eight atoms, but the problem is that these compounds are very reactive.
Question 11.
Draw the electron bond between two oxygen atoms.
Answer:

Question 12.
In electron dot structure, the valence shell electrons are represented by crosses or dots.
(a) The atomic number of chlorine is 17. Write its electronic configuration
(b) Draw the electron dot structure of chlorine molecule.
Answer:
(a) Electronic configuration of Cl (17):

(b) Electron dot structure of chlorine molecule:

Question 13.
Define hydrocarbon. Give two examples.
Answer:
A compound macle up of only hydrogen and carbon is called hydrocarbon. Methane (CR4), ethane (C2H4), etc.
Question 14.
Define saturated hydrocarbons.
Answer:
Hydrocarbons having single covalent bonds between the carbon atoms are called saturated hydrocarbons. For example, methane, ethane, etc.
Question 15.
Define unsaturated hydrocarbons.
Answer:
Hydrocarbons having double or triple covalent bonds between the carbon atoms are called unsaturated hydrocarbons. For example, ethene and ethyne.
![]()
Question 16.
What are alkanes? Give one example.
Answer:
Saturated hydrocarbons have single covalent bonds arid are called alkanes. For example, methane, ethane, etc.
Question 17.
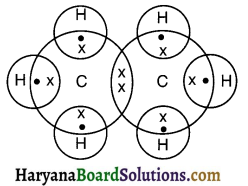
Draw electron dot structure of ethane.
Answer:
Ethane (C2H6):
Question 18.
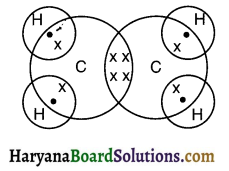
Draw electron dot structure if ethene.
Answer:
Ethene (C2H4):
Question 19.
What are alkenes and alkynes? Give one example.
Answer:
Unsaturated hydrocarbons have double or triple covalent bonds and are called alkenes and alkynes respectively. Ethane is alkene and ethyne is alkyne.
Question 20.
Which compound is shown here?
Answer:
Benzene
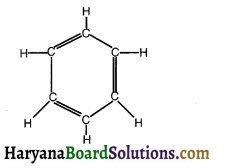
Question 21.
Name the following structure.
Answer:
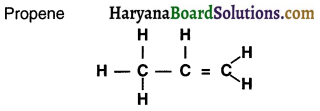
Question 22.
What is heteroatom?
Answer:
In an organic compound, any atom other than carbon or hydrogen is known as heteroatom.
Question 23.
What Is a functional group?
Answer:
An atom or a group of atoms (i.e. heteroatoms) responsible for chemical behavior of the parent molecule is called a functional group.
Question 24.
Mention the functional group containing oxygen.
Answer:
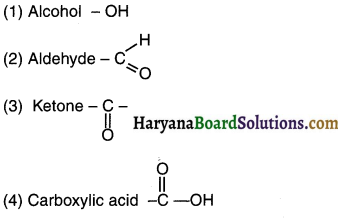
Question 25.
What is homologous series?
Answer:
The series of organic compounds in which each compound differs from its previous or later compound by (CH2) is called homologous series.
![]()
Question 26.
What is the difference between molecular masses of any two successive members of the homologous series? Why?
Answer:
Reason:The difference between two successive members of any homologous series is CH2. The atomic mass of carbon is 12 u and that of hydrogen is 1 u. So, the molecular mass of CH2 = 14 (12 x 1+1 x 2).
Question 27.
Why the physical properties of a compounds change as one moves in a homologous series?
Answer:
Physical properties are dependent on the molecular mass. Since, the molecular mass gradually increase in the series, the compounds show change in physical properties.
Question 28.
What do you mean by IUPAC names?
Answer:
Organic compounds or hydrocarbons have two names, (a) Common name and (b) IUPAC name. IUPAC names are names given by IUPAC i.e. International Union of Pure and Applied Chemistry. These names are also called IUPAC nomenclature.
Question 29.
Give common name and IUPAC name of any two compounds.
Answer:
| Common name | IUPAC name |
| (1) Methyl alcohol (2) Formic acid |
Methanol Methanoic acid |
Question 30.
How is the alcohol group represented?
Answer:
R-OH
Question 31.
What are aldehydes and ketones?
Answer:
Aldehydes and ketones are organic compounds having ( – CHO) and ( – C = O) respectively as their functional groups.
Question 32.
What are carboxylic acids?
Answer:
Organic compounds containing carboxyl (- COOH) groups as their functional group are called carboxylic acids.
Question 33.
Which compound is this:
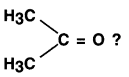
Answer:
Propanone
![]()
Question 34.
List four important properties of carboncorn pounds.
Answer:
(1) Combustion
(2) Oxidation
(3) Addition reaction arid
(4) Substitution reaction
Question 35.
Define complete combustion as a property of carbon compounds.
Answer:
Carbon and all its allotropes burn completely insufficient amount of oxygen. This is known as complete combustion.
Question 36.
Give the reaction when carbon burns insufficient supply of oxygen.
Answer:

Question 37.
Define oxidation of carbon compounds.
Answer:
Oxidation is the reaction in which carbon compounds take up oxygen in the presence of oxidizing agents to give another compound.
Question 38.
Define addition reaction.
Answer:
The reaction in which an unsaturated (alkene or alkyne) hydrocarbon combines with another substance to give a single but saturated (alkane) product is called addition reaction. Undergoing addition reaction is one of the properties of carbon compounds.
Question 39.
What is substitution reaction?
Answer:
The reaction in which one or more hydrogen atoms of a hydrocarbon are replaced by some other atoms (like chlorine) is called a substation reaction.
Question 40.
What is hydrogenation or hydrogenation of oils?
Answer:
When a vegetable oil (like groundnut oil) is heated with hydrogen in the presence of nickel catalyst, the oil turns into a saturated fat called vegetable ghee or say vanaspati ghee. This reaction is known as hydrogenation of oils.
Question 41.
State two uses of ethanol (alcohol).
Answer:
1. Ethanol is the active ingredient of all alcoholic drinks.
2. Ethanol is a good solvent and so it is also used to make medicines such as tincture iodine, cough syrups and several other tonics.
Question 42.
State the reaction of ethanol with sodium.
OR
A gas is evolved when ethanol reacts with sodium. Name the gas evolved and also write the balanced chemical equation of the reaction involved.
Answer:
Ethanol reacts with sodium and produces sodium ethoxide along with evolution of hydrogen gas.
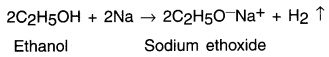
Question 43.
What is ethanoic acid?
Answer:
1. Ethanoic acid is the second member of the homologous series of carboxylic acids.
2. Its formula is CH3COOH. The common name of ethanoic acid is acetic acid.
![]()
Question 44.
Why in few countries a mixture of water and alcohol is used instead of simply water in car radiators?
Answer:
Water freezes at O °C. So, in countries where temperature falls below O°C mixing alcohol with water brings down the freezing point and car radiator can function property.
Question 45.
What is esterification?
Answer:
When ethanoic acid reacts with alcohols in the presence of little amount of concentrated sulphuric acid, the reaction produces esters. This reaction is called esterification reaction.
Question 46.
What is saponification?
Answer:
When the ester (CH3COOC2H5) formed in above reaction is heated with sodium hydroxide (a base) solution then the ester breaks down to give back original alcohol Le. ethanol and sodium salt of the carboxylic acid. This reaction is called saponification because it is used in making soap.
Question 47.
What is a soap molecule formed of?
Answer:
A molecule of soap is a sodium or potassium salt of long chain carboxiylic acid.
Question 48.
Give an idea of the hydrophlllc end of the soap.
Answer:
Hydrophilic end i.e. the polar head of a soap molecule is made up of functional group sodium carboxylate (-COONa). It is ionic in nature. Since it is hydrophilic it attracts water (or say dissolves in water).
Question 49.
Give a brief description of the non-polar tall of the soap.
Answer:
The non-polar tail is a long hydrocarbon chain. It is not Ionic. It is hydrophobic which means it repels water (i.e. it dissolves in oil). The hydrophobic carbon chain dissolves In oil or say dirt.
Question 50.
What are micelles?
Answer:
The soap molecule dissolves and get arranged in a systematic orientation where in the head portion faces towards the water and the tail faces the dirt. Due to this orientation, the oily dirt will get trapped at the centre of the micelle. Such patterns having ring like structures are called micelles. water brings down the freezing point and car radiator can function property.
Fill in the Blanks:
1. Acetic acid melting point : 290 K; Boiling point …………..
Answer:
391 K
2. The correct electron dot structure of a water molecule is …………..
Answer:
![]()
3. ………….. the chief constituent of natural gas.
Answer:
Methane
4. Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g. hydrogen. After the formation of four bonds. carbon attains the electronic configuration of ……………
Answer:
Neon
5. Covalently bonded molecules have ………….. intermolecular force.
Answer:
Weak
6. Covalent compounds have ……………. melting and …………… boiling points.
Answer:
Low; Low.
7. One of the allotrope of carbon is diamond. The other two are ………… and …………..
Answer:
Graphite; Buckminster fullerene.
8. As per an estimate there are about carbon compounds.
Answer:
3 million
![]()
9. Unsaturated compounds have bonds.
Answer:
Double or triple.
10. The first and simplest member of saturated hydrocarbon group is ……………
Answer:
Methane
11. Isomers are organic compounds having same formula but different formula.
Answer:
Molecular, structural
12. Almost all types of organic compounds have series.
Answer:
Homologous
13. Which out of alkyne, alkane and alkene is unsaturated?
Answer:
Alkene and alkyne
14. Chlorine, bromine and iodine belong to the functíonal group
Answer:
Halogen
15. The name of the compound CH3 — CH2 — CHO is …………….
Answer:
Propanal
16. Atomic mass of carbon is ……………….
Answer:
12u
17. The general formula of alkene is ……………….
Answer:
CnH2n
18. Kerosene will bum with ………………. type of flame.
Answer:
Yellow and sooty
19. We get a clean blue flame in our home stoves because of ……………….
Answer:
Burning saturated hydrocarbons In the presence of sufficient oxygen.
![]()
20. Substances capable of adding oxygen to others are called …………….
Answer:
Oxidizing agents
21. ….. and …… work as oxidizing alcohols to acids.
Answer:
Alkaline potassium permanganate; Acidified potassium dichromate.
22. Palladium and nickel work as ……………… in addition reaction.
Answer:
Catalysts
23. ………….. are substances that cause a reaction to occur or proceed at a different rate without affecting the reaction.
Answer:
Catalysts
24. Ideally, oils containing should be used for cooking.
Answer:
Unsaturated fatty acids.
25. ……………. an important compound is formed when sodium reacts with ethanol.
Answer:
Sodium ethoxide
26. Heating ethanol in at 443 K with excess concentrated sulphuric acid results in to give ethane.
Answer:
Dehydration of ethanol.
27. Because ethanoic acid freezes in winter it is also called …………..
Answer:
Glacial acid
28 hydrocarbons have a sweet fruity smell.
Answer:
Ester
29. Ester can be converted back into alcohol by treating with ……………
Answer:
Sodium hydroxide
30. Saponification reaction is used to prepare …………..
Answer:
Soap
31. Mast dirt of clothes is in nature.
Answer:
Oily
True Or False
1. Ethanol boils at 156 K. — False
2. Methanol boil at 111 K. — True
3. Nitrogen bonds with nitrogen through triple bond. — True
4. Carbon compounds are found in three shapes namely long chain, branched chain and elliptical rings.– False
5. The compounds of a homologous series show similar physical properties but different chemical properties. — False
6. For IUPAC nomenclature of Ketone compounds, the last alphabet ‘e’ is removed from the name of the hydrocarbon and the suffix ‘one’ is added. — True
7. The hydrocarbons in which any two nearby carbon atoms are combined by a double bond unsaturated hydrocarbons are called alkenes. — True
8. A molecule of detergent is ammonium or sulphonate salt of long chain carboxylic acki. — True
9. Soaps react with calcium and magnesium of hard water and then clean the clothes. — False
Match the Following
Question 1.
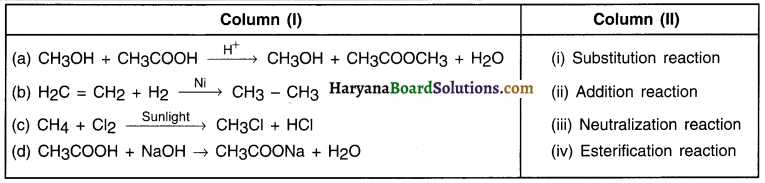
Answer:
(a-iv) (b-ii) (c-i) (d-iii)
![]()
Question 2.
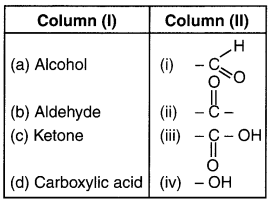
Answer:
(a-iv) (b-i) (c-ii) (d-iii)