Haryana State Board HBSE 7th Class Science Solutions Chapter 6 Physical and Chemical Changes Textbook Exercise Questions and Answers.
Haryana Board 7th Class Science Solutions Chapter 6 Physical and Chemical Changes
HBSE 7th Class Science Physical and Chemical Changes Textbook Questions and Answers
Question 1.
Classify the changes involved in the following processes as physical or chemical changes.
(a) Photosynthesis
(b) Dissolving sugar in water
(c) Burning of coal
(d) Melting of wax
(e) Beating aluminium to make aluminium foil
(f) Digestion of food.
Answer:
Physical Change (b), (d), (e)
Chemical Change (a), (c), (f)
Question 2.
State whether the following statements are true or false. In case a statement is false, write the corrected statement in your notebook.
(a) Cutting a log of wood into pieces is a chemical change.
(b) Formation of manure from leaves is a physical change.
(c) Iron pipes coated with zinc do not get rusted easily.
(d) Iron and rust are the same substances.
(e) Condensation of steam is not a chemical change.
Answer:
(a) False
(b) False
(c) True
(d) True
(e) True.

Question 3.
Fill the blanks in the following statements:
(a) When carbon dioxide is passed through lime water, it turns milky due to the formation of ………….. .
(b) The chemical name of baking soda is ……………… .
(c) Two methods by which rusting of iron can be prevented are …………. and …………. .
(d) Changes in which only …………. properties of a substance change are called physical changes.
(e) Changes in which new substances are formed are called …………. changes.
Answer:
(a) Calcium carbonate
(b) Sodium hydrogen carbonate
(c) Coating, galvanization
(d) Physical
(e) Chemical.
Question 4.
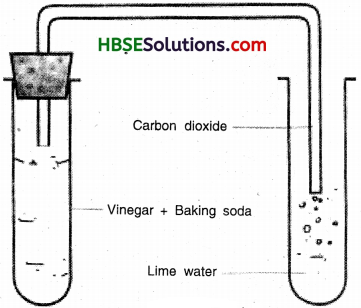
When baking soda is mixed with lemon juice, bubbles are formed with the evolution of a gas. What type of change is it? Explain.
Answer:
When baking soda is mixed with lemon juice, bubbles are formed with the evolution of a gas carbon dioxide.
lemon juice + baking soda → carbon dioxide + lime water
Since, a change in which one or more new substance are formed is called a chemical change, therefore this is a chemical change.
Question 5.
When a caùdie burns, both physical and chemical changes take place. Identify these changes. Give another example of a familiar process in which both the chemical and physical changes take place.
Answer:
Physical Change → Melting of ware
Chemical Change → Burning of candle
Question 6.
How would you show that setting of curd is a chemical change?
Answer:
The conversion of milk into curd, i.e., setting of curd is a permanent as well as irreversible and lead to the production of a new substance. The new substance, curd is formed has different composition and properties from the milk. Hence, setting of curd is a chemical change.
Question 7.
Explain why burning of wood is cutting it into small pieces are considered as two different types of a changes.
Answer:
Burning of wood is a chemical change because, in addition to new products burning is always accompanied by production of heat.
Cutting of wood into small pieces is a physical change because, pieces of wood underwent changes in size and no new substance is formed.
Question 8.
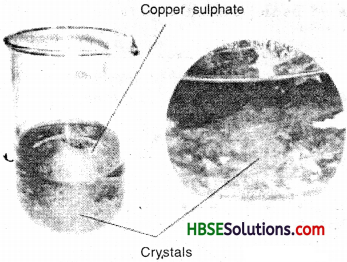
Describe how crystals of copper sulphate are prepared.
Answer:
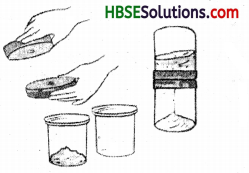
Take a cup full of water in a beaker and add a few drops of dilute sulphuric acid. Heat the water. When it starts boiling add copper sulphate powder slowly while stirring continuously. Continue adding copper sulphate powder till no more powder can be dissolved. Filter the solution. Allow it to cool. Do not disturb the solution when it is cooling. Look at the solution after some time. Now, you can see the crystals of copper sulphate.
Question 9.
Explain how painting of an iron gate prevents it from rusting.
Answer:
The process of rusting can be represented by the following equation:
Iron (Fe) + Oxygen (O2 from the air) + Water (H2O) → rust (iron oxide Fe2O3).
For rusting the presence of both oxygen and water (or water vapour) is essential. In fact, if the content of moisture in air is high, which means if it is more humid, rusting becomes faster. So, prevent iron gate from coming in contact with oxygen, or water, or both. One simple way is to apply a coat of paint or grease. In fact, these coats should be applied regularly to prevent rusting.

Question 10.
Explain why rusting of iron objects is faster in coastal areas than in deserts.
Answer:
The water of coastal areas contain many salts. The salt water makes the process of rust formation faster. Thus, rusting of iron objects is faster in coastal areas than deserts.
Question 11.
The gas we use in the kitchen is called liquified petroleum gas (LPG). In the cylinder it exist as a liquid. When it comes out from the cylinder it becomes a gas (Change – A) then it burns (Change – B). The following statements pertain to these changes. Choose the correct one.
(i) Process – A is a chemical change
(ii) Process – B is a chemical change.
(iii) Both processes A and B are chemical changes.
(iv) None of these processes is a chemical change.
Answer:
(iii) Both process A and B are chemical changes.
Question 12.
Anaerobic bacteria digest animal waste and produce biogas (Change – A). The biogas is then burnt as fuel (Change – B). The following statements pertain to these changes. Choose the correct one.
(i) Process – A is a chemical change,
(ii) Process – B is a chemical change.
(iii) Both process A and B are chemical changes.
(iv) None of these processes is a chemical change.
Answer:
(iii) Both process A and B are chemical changes.
Extended Learning – Activities And Projects
Question 1.
Take three glass bottles with wide mouths. Label them A, B and C. Fill about half of bottle A with ordinary tap water. Fill bottle B with water which has been boiled for several minutes, to the same level as in A. In bottle C, take the same boiled water and of the same amount as in other bottles. In each bottle put a few similar iron nails so that they are completely under water. Add a teaspoonful of cooking oil of the water in bottle C so that it forms a film on its surface. Put the bottles away for a few days. Take out nails from each bottle and observe them. Explain your observations.
Answer:
Do yourself.
Question 2.
Prepare crystals of alum.
Answer:
Do yourself.
Question 3.
Collect information about the types of fuels used for cooking in your area. Discuss with your teachers/parents/others which fuels are less polluting and why.
Answer:
Do yourself.

HBSE 7th Class Science Physical and Chemical Changes Important Questions and Answers
Very Short Answer Type Questions
Question 1.
What is a physical change?
Answer:
Change in which no new product is formed.
Question 2.
What is a chemical change?
Answer:
A change in which a new substance with different properties is formed.
Question 3.
Which of the following substance contains only one kind of atoms?
copper, iron, iron sulphide, sulphur, oxygen gas, water, air and hydrogen gas.
Answer:
The following contains only one kind of atoms:
Copper, Iron, Sulphur, Oxygen gas, Hydrogen gas.
Question 4.
Which of the following substances are compounds and which are elements?
Magnesium oxide, Mercuric oxide, Carbon, Nitrogen, Potassium permagnate, Sodium carbonate.
Answer:
Elements: Carbon, nitrogen.
Compounds: Magnesium oxide, mercuric oxide, potassium permanganate, Sodium carbonate.
Question 5.
Write the symbol of the following elements:
Aluminium, calcium, chlorine, cobalt, iodine and mercury.
Answer:
Al, Ca, Cl, Co, I, Hg.
Question 6.
Ne is the symbol of neon. What else does it represent?
Answer:
This symbol represent one atom of Neon.

Question 7.
Which elements are represented by the following symbols?
Na, K, P, Pb, Ca, Zn, Br, Sn.
Answer:
Sodium, Potassium, Phosphorus, Calcium, Zinc, Bromine, Tin.
Question 8.
Name four Elements which occur in gaseous form.
Answer:
Oxygen, Nitrogen, Hydrogen and Argon.
Question 9.
When some one open a bottle of perfumes, you smell it from a distance why it is so?
Answer:
It is due to the property of gases that their molecules diffuse (move) easily in the air.
Question 10.
What is crystallisation?
Answer:
The process of separation of pure crystals of a substance from its hot and supersaturated (concentrated) solution on cooling is called
crystallisation.
Question 11.
Define chemical reaction.
Answer:
The process in which the originally present substances change into new substances is called a chemical reaction.
Question 12.
What is matter?
Answer:
Anything which occupies space and has weight is called matter. Example: Air, Water, Wood, Stone, etc.
Question 13.
What are solids?
Answer:
Any materials which has a definite shape and definite volume, at room temperature is called solid.
Question 14.
What are liquids?
Answer:
Any substance which has a definite volume but no definite shape and has one free surface is called liquids: Milk, Water, Fruit juice, Alcohol etc.
Question 15.
Name two elements that are abundantly found in air.
Answer:
The two elements that are found abundantly in air are Nitrogen element and Oxygen element.
Question 16.
Choose the elements from the following substances: Marble, Mercury, Air, Carbon.
Answer:
Mercury and Carbon are elements.

Question 17.
What is the difference between 2N and N?
Answer:
2N represent 2 atoms of nitrogen and N represent 1 molecule of nitrogen.
Question 18.
Which of the following contains only one kind of atoms?
Copper, Iron sulphide, Sulphur, Oxygen gas, Water, Air and Hydrogen gas.
Answer:
Copper, Iron, Sulphur, Oxygen gas, Hydrogen gas.
Question 19.
Which of the following substances are compounds and which are elements?
Magnesium oxide, nitrogen, potassium permanganate, sodium carbonate.
Answer:
Compounds: Magnesium oxide, Potassium permanganate, Sodium carbonate.
Elements: Nitrogen.
Question 20.
Write down the chemical symbols of the following elements: Potassium, Calcium, Phosphorus, Nitrogen, Sulphur.
Answer:
K, Ca, P4, N and Sg.
Question 21.
Write the names of the elements having the following symbols: C, Br, P, Al, Si.
Answer:
Carbon, Bromine, Phosphorus, Aluminium and Silicon.
Question 22.
A given substance ‘X’ has definite shape, fixed volume, is in comprissible and non-diffusing. What is the physical state of the substance ‘X’?
Answer:
The substance ‘X’ is solid state.
Question 23.
Write the names of the elements which compose a molecule of water.
Answer:
Hydrogen and Oxygen.

Question 24.
Name the three most abundant elements on the earth’s crust.
Answer:
Iron, Aluminium and Silicon.
Question 25.
Give the chemical formulae of the following:
(i) Calcium hydroxide
(ii) Copper oxide
(iii) Iron chloride
(iv) Zinc nitrate
(v) Silver sulphate
(vi) Lead carbonate
(vii) Potassium phosphate
(viii) Sodium hydroxide
(ix) Hydrochloric acid
(x) Zince hydroxide.
Answer:
(i) Ca(OH)2
(ii) CuO
(iii) FeCl2
(iv) Zn (NO3)2
(v) Ag2SO4
(vi) PbCO3
(vii) K3PO4
(viii) NaOH
(ix) HCl
(x) Zn(OH)2.

Question 26.
Define reactents.
Answer:
The original substances that take part in a chemical reaction are called the reactants,
Question 27.
Define products.
Answer:
The substances that form as a result of chemical reaction are called the products.
Question 28.
Define chemical combination reaction.
Answer:
When two or more elements or compounds react chemically to form only one new product, then the reaction which takes place is called chemical combination.
Question 30.
Define chemical displacement reaction.
Answer:
When a more reactive element displaces a less reactive elements from its aqueous salt solution, the reaction which takes place is called chemical displacement.
Question 31.
What kind of chemical reaction takes place when a mixture of iron fillings are heated with sulphur?
Answer:
Chemical combination reaction.
Short Answer Type Questions
Question 1.
Formation of clouds is a physical change. Explain.
Answer:
Formation of clouds is a physical change. Clouds are formed by condensation of water vapours present in the atmosphere. When rainwater goes back on the earth no new product is formed. Therefore, it is a physical change.
Question 2.
Explosion of a cracker is a chemical change. Explain.
Answer:
When we burn a cracker, it exploide. Heat, light and smoke comes out after explosion. Many new products are formed. So, it is a chemical change.

Question 3.
Most physical changes are reversible. Give reasons with two examples.
Answer:
All physical changes are reversible. Because in physical changes, no new product is formed. They can be reversed easily.
Examples:
(i) Dissolving of sugar in water is a physical change and we get back sugar and water easily.
(ii) Formation of ice from water. In melting of ice, we can get water back.

Question 4.
Identify the type of change and state whether energy is evolved or absorbed in each one of the following:
Burning of a candle, lighting of a bulb, preparation of food by green plants, Volcanic eruption.
Answer:
(i) Burning of a candle: Chemical change, energy evolved.
(ii) Lighting of a bulb: Physical change, energy evolved.
(iii) Preparation of food by green plants: Chemical change, energy absorbed.
(iv) Volcanic eruption: Chemical change, energy evolved.
Question 5.
What is a chemical formula? What information does it provide?
Answer:
Chemical formula is a shorthand method of using symbols to represent the composition of a compound. Using it, we can get the following informations:
(i) Constituting name of the elements present in the compound, e.g., water has the formula H20. It shows that water is made up .. of two elements, hydrogen and oxygen.
(ii) Atoms present in each element, e.g.,
(iii) The composition of compound and the formula H2O shows that in a molecule of molecular weight of the compound, this compound two atoms of hydrogen and one atom of oxygen are present.
Question 6.
What are the differences between chemical and physical changes?
Answer:
Differences between chemical and physical changes:
| Chemical change | Physical change |
| 1. A new substance is formed. | 1. No new substance is formed. |
| 2. It is a permanent change. | 2. It is a temporary change. |
| 3. The composition of new substances changes. | 3. No change in the composition of change takes place. |
| 4. It is irreversible. | 4. It is reversible. |
| 5. Heat/light evolved or absorbed during change. | 5. No heat light evolved or absorbed or may be evolved or absorbed. |
Question 7.
How is common salt obtained from sea water?
Answer:
In the coastal regions of India, especially in the Rann of Kutch in Gujarat and some parts of Tamil Nadu, the sea water is collected in shallow pits. It is then allowed to evaporate in the sun. As the water evaporates, the salt solution becomes supersaturated. This supersaturated solution cannot hold the excess salt. Thus, it separates out in form of salt crystals. These salt crystals are collected. They are redissolved in water and the solution is filtered to remove insoluble impurities. The clear solution is again evaporated so as to obtain the crystals of pure salt.
Question 8.
What is crystallisation? To what purpose is it put?
Answer:
The process of separation of pure crystals of a substance from its hot and supersaturated (concentrated) solution on cooling is called Crystallisation.
The process of crystallisation is employed for the separation of a pure water soluble substance from its mixture. For example, if there is a mixture of alum and common salt, the pure alum crystals can be separated by the process of crystallisation.

Question 9.
What is the significance of an equation?
Answer:
Significance of an equation:
(i) It tells us which substance reacted and which substances are produced.
(ii) It tells us the quantities of each of the reactants and each of the products.
Question 10.
Give four examples of a physical change.
Answer:
(i) Formation of dew.
(ii) Evaporation of water.
(iii) Melting of wax.
(iv) Making of ice-cream.
Question 11.
Give four examples of a chemical change.
Answer:
(i) Photosynthesis by plants.
(ii) Clotting of blood.
(iii) Curdling of milk.
(iv) Burning of candle.
Question 12.
State four characteristics of physical change.
Answer:
(i) It is temporary.
(ii) It is reversible.
(iii) No new substance is produced.
(iv) No change in the chemical properties.
Question 13.
State four characteristics of chemical change.
Answer:
(i) It is permanent.
(ii) It is irreversible.
(iii) New substance is produced.
(iv) Changes in the chemical properties due to the formation of new substances.
Question 14.
What happens when an iron blade of a knife is dipped in copper sulphate solution? What kind of chemical reaction takes place?
Answer:
When an iron blade of a knife is dipped in copper sulphate solution iron blade is coated with reddish deposit of copper. Thus, we can say that iron (more reactive element), displaces copper (less reactive element), from its aqueous copper sulphate solution. Chemical displacement reaction is takes place.
Question 15.
Why do the molecules in a liquid tend to stay together and give a condensed form?
Answer:
In a liquid, the molecular motion are not great enough to over come the force of attraction between molecules. That is why the molecules tend to stay together and give a condensed form.
Question 16.
On a hot summer day the cycle tubes burst suddenly. Explain why?
Answer:
The cycle tubes are filled with air. On a hot summer day, the temperature of the atmosphere is high. When cycle runs on the road, the friction between the cycle wheel and the path produces heat which raises the temperature of the air in the cycle tubes. On heating the air expands and exerts pressure in the inside of the tubes. Due to the pressure, the cycle burst suddenly on a hot summer day.

Question 17.
Why can the same substance exist in all the three states, that is, solid liquid and gas?
Answer:
The space between the molecules, the force of attraction between the molecules, and the amount of movement of the molecules of a substance can be changed by changing the pressure and temperature of the substance. So depending on the pressure and temperature, the same substance can exist in all the three states i.e., Solid, liquid and gaseous. For example under normal pressure water exists as a solid in the form of ice at a temperature of 0°C or below. It exists as a liquid in the form of water at the room temperature and as a gas in the form of steam at 100°C or above.
Question 18.
Write some important properties of an element.
Answer:
Properties of an element are:
(i) An element is made up of some kinds of atoms.
(ii) An element cannot be Broken up into much simpler substances,
(iii) An element can be represented by a chemical symbol.
Question 19.
What are the important characteristics of a compound?
Answer:
(i) Chemical compound is made up of two or more elements combined chemically.
(ii) They have fixed formula or composition.
(iii) They have fixed melting point (M.P.) and boiling point (B.P.).
(iv) Energy is either evolved or absorbed during formation of a compound.
(v) Properties of constituent elements are different from its compound, e.g., properties of water (H2O) are different from properties of hydrogen and oxygen.
Question 20.
What does the formula CO2 represent?
Answer:
Molecular formula CO2 represents:
(i) the name of the compound carbon dioxide.
(ii) carbon dioxide is made up of two kinds of elements – carbon and oxygen.
(iii) one molecule of carbon dioxide has one atom of carbon and 2 atoms of oxygen.
(iv) the molecular mass of carbon dioxide is 12 + 2 x 16 = 44 gram.
Question 21.
How is salt obtained from sea-water?
Answer:
The seas are great sources of salts. A litre of a water contains about 35 grams of salts. Sodium chloride is the main salt. The sea-water is trapped in shallow called lagoons and is allowed to evaporate in sunlight to white solid crystal of salts. These crystals are processed and packed to send the markets.
Question 22.
Give three reasons for supporting that water is a compound and not a mixture.
Answer:
Water is considered a compound due to the following reasons:
(i) Water cannot be separated into its constituents, hydrogen and oxygen, by the physical process.
(ii) The properties of water are entirely different from those of its constituents hydrogen and oxygen.
(iii) Water contains hydrogen and oxygen combined together in a fixed proportions of 1:8 by weight.
Question 23.
Explain why solution of salt in water is considered a mixture and not a compound. Give three reasons.
Answer:
Salt solution is considered a mixture due to the following reasons:
(i) Salt solution can be separated into salt and water by the physical process.
(ii) Salt solution, shows the properties of both its constituents salt as well as water.
(iii) The composition of salt solution is variable i.e., the percentage of salt and water in different salt solutions are different.

Question 24.
What information do we get from a chemical equation?
Answer:
The informations obtained from a chemical equation are:
(i) Name of the substance (elements or compound) taking part in reaction, i.e., reactants and products.
(ii) The number of atoms and molecules of different substances.
(iii) The conditions under which the reaction takes place or taking part in reaction, for example:
N2 + 3H2 → 2NH3
It represents 1 molecule of nitrogen combines with 3 molecules of hydrogen to form 2 molecules of ammonia.
Question 25.
“In a chemical reaction, there is only a rearrangement of the atoms of the reactants.” Explain this statement giving an example.
Answer:
In a chemical reaction, there is only a x’e. angement of the atoms of the reactants because when a chemical reaction occurs new products are formed. These products have same kind and number of atoms as are present in reactants. They are only rearrange to give new substances. For example, in a reaction between iron and sulphur to give iron sulphide the number of atoms of iron and sulphur are identical on both sides of the equation.
Fe + S → FeS (On heating)
Question 26.
What is done to make a chemical equation more informative?
Answer:
To make a chemical equation more informative it should be balanced and conditions of the reaction should be written above the arrow. For example,

It shows that 2 molecules of water on electrolysis break up into 2 molecules of hydrogen and 1 molecule of oxygen.
Question 27.
What do you understand by reactants and products?
Answer:
Reactants: Substances taking part in a reaction are called reactants. They are written on the left side of the chemical equation.
Products: Substances produced in a reaction are called products. They are written on the right side of the equation.
Example:

Long Answer Type Questions
Question 1.
What does a chemical formula represent?
Answer:
Significance of chemical formula:
(i) It represents the name of the substance.
(ii) It represents one molecule of the substance.
(iii) It gives the names of all the elements present in the molecule.
(iv) It represents the mass of one molecule.
As an example of the formula of H2SO4:
(i) Represents sulphuric acid.
(ii) Represent one molecule of sulphuric acid.
(iii) Tells that sulphuric acid contains three elements:
hydrogen, sulphur, and oxygen.
(iv) Tells that one molecule of sulphuric acid contains two atoms of hydrogen; 1 atom of sulphur and 4 atoms of oxygen.

Question 2.
What do you understand by the following terms? Give examples.
(i) Endothermic chemical change
(ii) Exothermic chemical change.
Answer:
(i) Endothermic chemical change: When a chemical change takes place with the absorption of heat energy, then the change is said to be endothermic. .
Examples: Heating of mercuric oxide to form mercury and oxygen. Heating of calcium carbonate to form calcium oxide and carbon dioxide.
(ii) Exothermic chemical change: When a chemical change takes place with the liberation of heat energy, the change is said to be exothermic.
Examples: Candle on burning liberates heat and light energy. Respiration is an exothermic change.
Question 3.
What is a compound? How does it differ from a mixture?
Answer:
A compound consists of two or more elements, joined together in a fixed ratio by chemical bonds. For example, water formed from hydrogen and oxygen, sodium chloride formed from sodium and chlorine, sugar formed from carbon, hydrogen and oxygen, nitre formed from potassium, nitrogen and oxygen are all compound.
Difference between compounds and mixtures:
| Compound | Mixture |
| (i) A compound consists of only tne kind of chemical substance. | (i) A mixture consists of two or more chemically different substances. |
| (ii) The components of a compound cannot be separated by a simple means. | (ii) The composition of mixture can be separated easily by simple means. |
| (iii) Heat, light or electricity is absorbed or evolved during its formation. | (iii) Its formation is not accompanied by absorption or evolution of heat, light or electricity. |
| (iv) Compound has definite formula. | (iv) Mixture do not have definite formula. |
| (v) Compounds have definite M.P. and B.P. Compound is always homogeneous | (v) Mixture do not have fixed M.P. and B.P. |
Question 4.
What is the difference between a balanced and unbalanced chemical equations? Explain with an example.
Answer:
Difference between a balanced and unbalanced chemical equations:
In unbalanced chemical equations, the number of each element atoms do not same. As for example:
Mg + O2 → MgO.
This is not a balanced equation because the number of Mg atom and oxygen atoms are not same on both the sides, Le., reactants and product sides. On the other hand, in a balanced chemical equation the number of each atoms of each elements on both sides should be equal. As for example:
2Mg + O2 → 2MgO.
In this balanced chemical equation the number of magnesium atoms and oxygen atoms in the left hand side are equal to the right hand side of the equation. So it is a balanced chemical equations.
(i) A mixture consists of two or more chemically different substances.
(ii) The composition of mixture can be separated easily by simple means.
(iii) Its formation is not accompanied by absorption or evolution of heat, light or electricity.
(iv) Mixture do not have definite formula.
(v) Mixture do not have fixed M.P. and B.P.
(vi) Mixture can be homogeneous and heterogenous.
Question 5.
Balance the following equations:
(i) Fe + O2 → Fe2O3
(ii) H2O + H2 → O2
(iii) Mg + O2 → MgO
(iv) Al + O2 → Al2O3
(v) Fe + HCl → FeCl3 + H2
(vi) Cu + O2 → CuO
(vii) Hg + O2 → HgO
(viii) Zn + HCl → ZnCl2 + H2
(xi) Al + HCl → AlCl3 + H2
(x) N2 + H2 → NH3
Answer:
(i) 4Fe + 3O2 → 2Fe2O3
(ii) 2H2O → 2H2 + O2
(iii) 2Mg + O2 → 2MgO
(iv) 4Al + 3O2 → 2Al2O3
(v) 2Fe + 6HCl → 2FeCl3 + 3H2
(vi) 2Cu + O2 → 2CuO
(vii) 2Hg + O2 → 2HgO
(viii) Zn + 2HCl → ZnCl2 + H2
(xi) 2Al + 6HCl → 2AlCl3 + 3H2
(x) N2 + 3H2 → 2NH3

Physical and Chemical Changes Class 7 HBSE Notes
1. Properties such as shape, size, colour and state of a substance are called its physical properties.
2. A change in which a substance undergoes a change in its physical properties is called a physical change. A physical change generally reversible.
3. A change in which one or more new substances are formed is called a chemical change. A chemical change is also called a chemical reaction.
Some common examples of chemical change:
1. Burning of wood or charcoal.
2. Burning of Candle.
3. Decomposition of water into hydrogen and oxygen.
4. Formation of water from hydrogen and oxygen.
5. Digestion of food.
6. Curdling of milk.
7. Formation of biogas (Crobar gas).
8. Burning of petrol or diesel.
9. Smoking of cigarette.
10. Drying of paint.
11. Rusting of iron.
12. Ripening of fruit.
13. Baking of cake.
14. Photosynthesis by plants.
15. Formation of wine.
Some Common Examples of Physical Changes:
1. Formation of dew.
2. Evaporation of water.
3. Crystallisation of sugar from its solution.
4. Ringing of an electric bell.
5. Breaking of glass pane.
6. Making of ice-cream.
7. A rock rolling down a hill.
8. Bending of glass tube by heating.
9. Melting of wax.
10. Sublimation of camphor.
4. If you leave a piece of iron in the open for some time, it acquires a film of brownish substance. This substance is called rust and the process is called rusting.
5. Any pure substance which cannot be broken into two or more pure substances by any chemical means is called an element.
6. The smallest unit of an element, which takes part in a chemical reaction is called an atom.
7. The smallest unit of a pure substance, which always exists independently and can retain physical and chemical properties of that substance, is called a molecule.
8. A metal is an element which is generally malleable, ductile and a good conductor of heat and electricity. About 80% of the elements are metals.
9. Non-metals are bad conductors of heat and electricity. They are neither malleable nor ductile. Non-rr Hals are generally soft.
10. When the molecule of a substance contains two or more atoms of different elements, combined together in a definite ratio, then it is said to be a molecule of a compound.
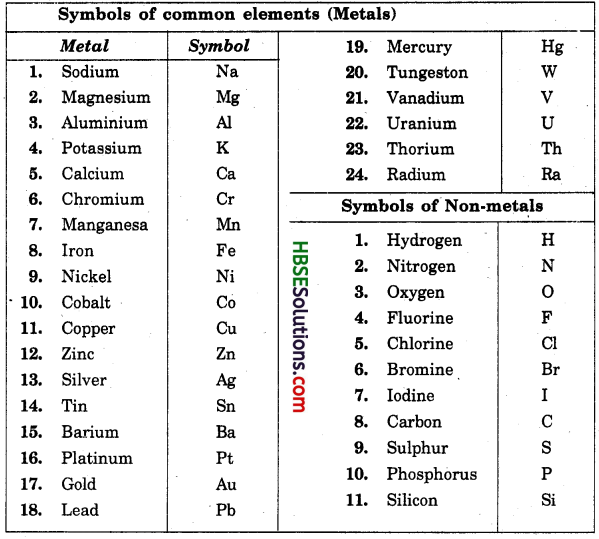
Symbols of common elements (Metals)
11. When one or more substances (elements or compounds) undergo a chemical change, with the absorption or release of energy, so as to form one or more new products, then the change taking place collectively is called chemical combination.
12. When two or more elements or compounds react chemically to form only one new product, then the reaction which takes place is called chemical combination.
13. When a single chemical compound decomposes on heating or by some other kind of energy, so as to form two or more new substances (elements or compounds), then the chemical reaction which takes place is called chemical decomposition.
14. When a more reactive element displaces a less reactive element from its aqueous salt solution, the reaction which takes place is called chemical displacement.
15. When an acid solution reacts with a base or metal carbonate, so as to form a salt, then the reaction is called neutralisation reaction.
16. The process of separation of pure crystals of a substance from its hot and supersaturated (concentrated) solution on cooling is called crystallisation.
Read More:
Gift Nifty Indices
![]()
![]()
![]()








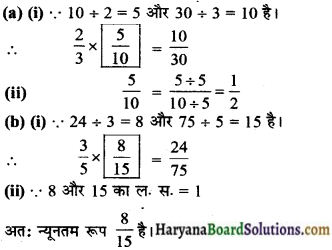




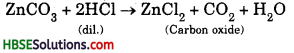
 Soil profile
Soil profile