Haryana State Board HBSE 10th Class Science Notes Chapter 3 Metals and Non-metals Notes.
Haryana Board 10th Class Science Notes Chapter 3 Metals and Non-metals
Physical Properties of metals
Metals: Those elements which lose electrons to become positive ions are called metals. For example, magnesium (Mg) is a metal since it loses 2 electrons to become positive ion Mg2+.
Physical properties of metals:
- Luster
- Hardness
- Malleability
- Ductility
- Conductivity of heat
- Melting and boiling points
- Electrical conductivity
- Sonorous and
- Alloys.
Physical Properties of Non-metals
Non-metals:
Elements that form negative ions by gaining electrons are called non-metals. For example, oxygen forms oxide ion O-2 by gaining electron and hence oxygen is a non-metal.
![]()
Allotrope:
Some elements possess the property of existing in two or more different forms in the same physical state. Such property is called allotropy. The different forms of the elements are called allotropes. Example : Carbon
What Happens when Metals are Burnt in Air:
Metals combine with oxygen to form metal oxides.
Metal + Oxygen gas → Metal oxides
Amphoteric oxides: Some metal oxides react with both acids and bases to produce salt and water. Such metal oxides are called amphoteric oxides.
Reaction of metal with water (H2O):
Metals on reaction with water form metal oxides and produce hydrogen gas. Metal oxides that are soluble in water dissolve in it to further form metal metal hydroxide.
Metal + Water → Metal oxide + Hydrogen gas + Heat Metal oxide + Water → Metal hydroxide
Reaction of Metals will, Acids
Those metals which react with dilute acids produce metal salt and hydrogen gas.
Metal + Dil. acid → Salt corresponding to metal + Hydrogen gas
Reaction of Metals with Solutions of Other Metal Salts
When metals react with solutions of salts of other metals, the more reactive metals displace less reactive metals from their salt solutions.
Metal A + Salt solution of metal B → Salt solution of metal A + Metal B
The Reactivity Series
Activity (reactivity) series: The arrangement of metals in decreasing order of their reactivities is called the activity or reactivity series of metals.
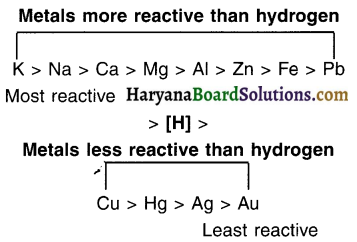
Activity series : Relative reactivities of metals
![]()
Information that we get from reactivity series of metals:
- A more reactive metal will displace a less reactive metal (i.e. a metal below it in the reactivity series) from its solution.
- Metals present at the top of the activity series are less electropositive and do not occur freely in nature. On the other hand, metals at the bottom of the series are more electropositive and generally occur freely in nature.
How do Metals and Non-metals React
Chemical bond : The phenomenon through which atoms of a molecule of a compound attract each other and combine is known as a chemical bond.
Chemical bonds are of two types. They are:
- Ionic bond: The bond formed between a metal and a non-metal is called an ionic bond. For example, sodium (Na) metal and chlorine (Cl) non-metal bond with each other through ionic bond and form NaCl (common salt).
- Covalent bond: The bond formed between two non-metals is called a covalent bond. For example, two hydrogen (H) atoms join with each other through a covalent bond and form a hydrogen molecule (H2).
![]()
Properties of ionic compounds
- Physical nature: Ionic compounds are obtained in solid form. They are hard and brittle.
- Melting point and boiling point: Their melting and boiling points are high.
- Solubility: They are soluble in water but insoluble in organic solvents such as kerosene, petrol, etc.
- Conduction of electricity: An ionic compound cannot conduct electricity in solid form.
Occurrence of Metals:
Mineral:
The elements or compounds that occur naturally in the earth’s crust are called minerals. Sea-water also contains salts of metals such as sodium chloride, magnesium chloride, etc.
Ores:
Those minerals from which the metals can be extracted, conveniently and profitably are called ores.
Gangue:
Impurities such as sand, mud, etc. present in the ore are called gangue.
![]()
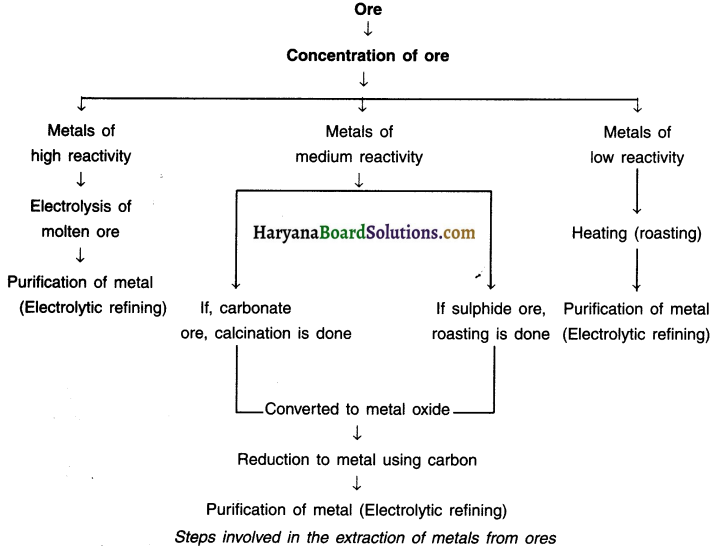
Metal occur in the earth in the following forms:
- In free state (Least reactive metals). For example, silver (Ag), gold (Au) and platinum (Pt).
- In the form of compounds (Highly reactive metals). For example, potassium (K), sodium, Magnesium (Mg), Aluminium (AI), etc.
- In the form of oxides/sulphates/carbonates (Moderately reactive metals). For example, zinc (Zn), Iron (Fe), Lead (Pb),
Steps Involved in extracting metal from its ore:
Corrosion:
Corrosion: The erosion reaction that takes place between a metal and water or moisture when they come in contact with each other is called metallic erosion or corrosion.
Ways to prevent corrosion of iron:
- Galvanizing: The process of applying zinc is called galvanizing and the iron on which it is applied is then called galvanized iron.
- Make alloys: An alloy is a homogeneous mixture of two or more metals or metal and non – metal. Stainless steel, brass, gold jewellery, etc. are alloys.