Haryana State Board HBSE 8th Class Science Solutions Chapter 6 Combustion and Flame Textbook Exercise Questions and Answers.
Haryana Board 8th Class Science Solutions Chapter 6 Combustion and Flame
HBSE 8th Class Science Combustion and Flame Textbook Questions and Answers
Question 1.
List conditions under which combustion can take place.
Answer:
Following conditions are necessary for combustion:
(i) The substance should be combustible.
(ii) It should have low ignition temperature.
(iii) There should be proper supply of air.
Question 2.
Fill in the blanks:
(a) Burning of wood and coal causes …………. of air.
(b) A liquid fuel, used in homes is …………. .
(c) Fuel must be heated to its before it …………. starts burning.
(d) Fire produced by oil cannot be controlled by …………. .
Answer:
(a) pollution
(b) Kerosene
(c) ignition temperature
(d) water.
Question 3.
Explain how the use of CNG in automobiles has reduced pollution in our cities.
Answer:
CNG has replaced petrol and diesel as fuel in automobiles because petrol and diesel produced a lot of unbumt carbon particles and emitted carbondioxide and nitrogen oxides and sulphur dioxide. These all gases are poisonous gases and cause various environmental hazards. But CNG is safe because it produces these substances in very small amounts thus reducing pollution in cities.
![]()
Question 4.
Compare LPG and wood as fuels.
Answer:
| LPG | Wood |
| It has more calorific value i.e. 55000 kJ/kg. It is smoke free fuel. | It has less calorific value i.e. 17000 to 22000. |
| It is easy to transport. | It gives out lot of smoke which is quite dangerous. |
| It is easily stored in cylinders. | It is difficult to transport wood. |
| It does not cause any environment problem. | It is difficult to store as it needs of space to store. It is cut so lead to deforestation thus gives rise to many natural and environmental problem. |
Question 5.
Give reasons:
(a) Water is not used to control the fire involving electrical equipment.
(b) LPG is a better domestic fuel than wood.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not. .
Answer:
(a) Water is a conductor of electricity, so it can easily conduct electric current and causes danger of electric shocks.
(b) LPG is a better domestic fuel than wood because unlike wood it does not produce smoke and is comparatively easy to transport.
(c) Paper by itself catches fire easily because it has low ignition temperature but when wrapped around an aluminium pipe its
Question 6.
Make a labelled diagram of a candle flame.
Answer:
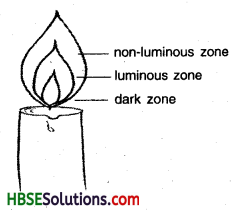
Different Zones of Candle flame
Observe the foaming reaction. What happens to the candles? Why? In what order?
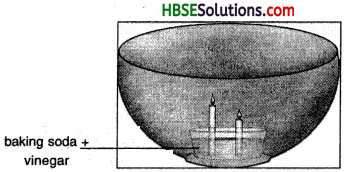
Answer:
The candles get extinguished. The smaller candle with get extinguisher first because thfe supply of oxygen is cut of due to foam; The smaller candle will come in effect of foam earlier than the longer one and thus stop burning prior to the longer candle.
Activity
Combustible and Non-combustible Substances
| Material | Conmbustible | Non-combustible |
| Wood | ✓ | |
| Paper | ✓ | |
| Iron nails | ✓ | |
| Kerosene oil | ✓ | |
| Stone piece | ✓ | |
| Straw | ✓ | |
| Charcoal | ✓ | |
| Matchsticks | ✓ | |
| Glass | ✓ |
HBSE 8th Class Science Combustion and Flame Important Questions and Answers
Very Short Answer Type Questions
Question 1.
Why do we need fuel?
Answer:
We need fuel to generate energy.
Question 2.
Name any three fuels.
Answer:
Coal, wood, LPG, kerosene, petrol.
Question 3.
What is produced during combustion?
Answer:
Heat and light.
Question 4.
In what forms light is given during combustion?
Answer:
In form of flame or glow.
Question 5.
How does charcoal burn?
Answer:
It burns with glow.
Question 6.
How does a candle burn?
Answer:
It burns with flame.
![]()
Question 7.
Name two objects which burn without flame.
Answer:
Coal and charcoal.
Question 8.
Name two objects which burn with flame.
Answer:
LPG and candle.
Question 9.
Name any three combustible substances.
Answer:
Wood, coal, LPG.
Question 10.
What is necessary for substances to burn?
Answer:
Air.
Question 11.
How does sun produces heat although it does not have air?
Answer:
It produces heat by nuclear reactions.
Question 12.
In addition to air, what else is a necessary condition for a substance to burn?
Answer:
Low ignition temperature.
Question 13.
What are the substances with very low ignition temperature called?
Answer:
Inflammable substances.
Question 14.
Name some inflammable substances.
Answer:
Alcohol, petrol, LPG., etc.
![]()
Question 15.
What substances are used to extinguish fire?
Answer:
Water, sand, fire extinguishers.
Question 16.
What should we use to extinguish fire in case of electric short circuit?
Answer:
Sand or soil.
Question 17.
Name different types of fire extinguishers.
Answer:
(i) Soda-acid fire extinguishers
(ii) Hydrocarbon fire extinguisher.
Question 18.
Name different types of combustions.
Answer:
Rapid combustion, Spontaneous combustion and explosions.
Question 19.
Name different zones of a flame.
Answer:
Outer non-luminous zone, luminous zone and dark zone.
Question 20.
Which zone of a flame has highest temperature?
Answer:
Non-luminous zone.
Question 21.
Name two properties of an ideal fuel.
Answer:
High calorific value and low cost.
Question 22.
Is there any ideal fuel?
Answer:
No ideal fuel exist.
Question 23.
What is smoke?
Answer:
Smoke is unburnt carbon particles.
Question 24.
Which diseases do incomplete combustion cause?
Answer:
Respiratory and skin diseases.
![]()
Question 25.
What is supposed to be the cause of global warming?
Answer:
Increasing amount of carbon dioxide.
Question 26.
What is the rise in temperature of the environment of earth called?
Answer:
Global warming.
Question 27.
What does global warming lead to?
Answer:
Global warming leads to melting of polar glaciers.
Question 28.
Which chemicals give rise to acid rains?
Answer:
Sulphur dioxide and nitrogen oxides.
Question 29.
Which fuel is being used in automobiles in place of petrol and diesel?
Answer:
CNG.
Question 30.
Does CNG produce poisonous substances on burning?
Answer:
In very small amounts.
Short Answer Type Questions
Question 1.
Define combustion.
Answer:
A chemical process in which a substance reacts with oxygen to give off heat is called combustion. Burning of substances to get heat is called combustion.
Question 2.
What is produced when a combustible substance burns?
Answer:
When a combustible substance burns it produces heat and light. Light is in the form of glow or flame. Some combustible substances do not
burn in a flame, they simply glow.
Question 3.
How will you prove that air is necessary for burning?
Answer:
Burn a candle. Now put a beaker inverted on it. You will see the flame will flicker and then it will extinguish. As the inverted beaker has cut off the oxygen supply, the flame is extinguished. This proves air is necessary for burning.
Question 4.
What do you mean by ignition temperature?
Answer:
The lowest temperature at which a substance catches fire is called its ignition temperature. This is the temperature which has to be there, if the substance has to burn, below it the object will not catch fire.
Question 5.
What are inflammable substances?
Answer:
Some substances have very low ignition temperature and they catch fire very easily. Suflj substances are called inflammable substances. Petrol, alcohol, LPG etc. are the inflammable substances.
![]()
Question 6.
Write three things necessary for fire to produce?
Answer:
Three conditions necessary to produce fire are following:
(i) Presence of oxygen i.e, air.
(ii) Ignition temperature.
(iii) Presence of combustible substances.
Question 7.
What can be done to put the fire off?
Answer:
To put off the fire one of the conditions necessary for burning should be removed or controlled. If we cut off the supply of air, fire will be extinguished or we can bring the ignition temperature down to blow off the fire.
Question 8.
Why can’t water control the fire due to oils?
Answer:
Water cannot put off fire due to oils because water is lighter than oil. It settles down the oil particles and cannot bring the ignition temperature of the substance down, thus cannot control the fire.
Question 9.
What should be done to control fire due to oils?
Answer:
In case of fire due to oils, water is not useful as it is lighter than oil and settles down below the oil particles. In such cases, sand or soil should be used. They cut off the supply of air to the fire and it is put off.
Question 10.
How does carbondioxide help in putting off fire?
Answer:
Carbondioxide covers the surface of the burning substance and does not allow the oxygen to reach the substance. In absence of oxygen the fire gets put off.
Question 11.
What is rapid combustion?
Answer:
When gases bum quickly to produce heat and light it is called rapid combustion. This process takes place rapidly and takes less time.
Question 12.
What is spontaneous combustion?
Answer:
When an object catches fire on its own without any apparant cause, it is called spontaneous combustion. In this type of combustion the object suddenly bursts into flame. For example, phosphorus catches fire in air.
Question 13.
Write the properties of an ideal fuel.
Answer:
An ideal fuel has following qualities:
(i) It has high calorific value.
(ii) It has low ignition temperature.
(iii) It is cheap and easily available.
(iv) It can be easily transported.
Question 14.
Write some harmful effects of burning fuel.
Answer:
Burning fuel can cause following ill effects:
(i) Unbumt carbon particles of these fuels can cause respiratory and skin diseases.
(ii) Smoke emitted by these fuels cause pollution and many diseases.
(iii) Poisonous gases like carbondioxide, carbonmonoxide and sulphurdioxide etc. pollute the air.
![]()
Long Answer Type Questions
Question 1.
Explain the conditions necessary for cpmbustion.
Answer:
Combustion is burning of substances. For combustion of substances following conditions are necessary: .
(i) Air:
Substances only bum in sufficient supply of air i.e. oxygen. If the supply of air is hindered the object will not bum. In case of a burning object, if air is hindered, it will stop burning. This fact is used in extinguishing the fires.
(ii) Ignition Temperature:
Ignition temperature is the minimum temperature at which a substance starts burning. Those substances which have low ignition temperature can bum easily, while those which have high ignition temperature need more heating and burn late.
(iii) Presence of combustible substance:
If a substance is not combustible, it will not catch fire at all, so to produce fire it is necessary to have a combustible substance.
Question 2.
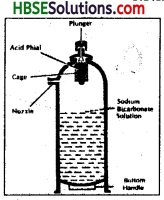
Describe the working of soda-acid fire extinguisher.
Answer:
Soda-acid fire extinguisher is based on the cooling the temperature of the burning object. This type of fire extinguisher contains sulphuric acid and sodium bicarbonate. When the extinguisher becomes functional, the sulphuric acid reacts with sodium bicarbonate to produce carbondioxide. Carbondioxide when released, cuts off the supply of oxygen and water is released to bring down the ignition temperature of the burning object. In this way fire is controlled.
Question 3.
What are different types of combustion?
Answer:
Combustion is of three types:
(i) Rapid combustion: When gases bum rapidly to produce heat and light it is called rapid combustion.
(ii) Spontaneous combustion: When any material like phosphorus bums on its own without any apparant cause, it is called spontaneous combustion.
(iii) Explosion:
When combustion takes place with sudden release of heat and light and a large amount of gas in form of bang, it is called explosion as in case of crackers and bombs.
![]()
Question 4.
Explain various harmful effects of burning fuels.
Answer:
Fuels are very useful for us. But there are certain disadvantages of burning fuels.
(i) Fuels like wood, coal, petroleum etc. which are carbon fuels generally release unbumt carbon particles which are quite harmful. They cause various respiratory and skin diseases.
(ii) In most of the cases carbondioxide is released during burning of fuels. This carbondioxide is released in environment. Carbondioxide is an identified cause of increasing temperature of the earth, which is called global warming.
(iii) Incomplete combustion of fuels release many poisonous gases like carbonmonoxide. in the environment. These poisonous gases create air pollution, such air if inhaled can be fatal.
(iv) Combustion of coal and diesel generates sulphurdioxide. It is a corrosive gas and it causes suffocation.
(v) Burning of petrol releases nitrogen oxides. These oxides of nitrogen and sulphurdioxide combine with rain water to cause acid rains.
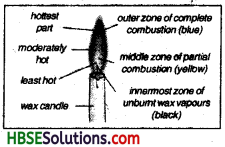
Question 5.
Describe the various zones of a candle flame.
Answer:
The candle flame has three different zones. These zones can be distinguished on the basis of their colour.
(i) The outer zone:
It is blue in colour and it has very high temperature. It is the hottest zone of the flame. In this zones the wax vapours bum completely due to availability of enough oxygen, in turn carbondioxide and water vapours are produced.
(ii) The middle zone:
In this zone the flame bums with yellow colour and the wax vapours start burning here. The yellowish colour of the flame is due to the burning of carbon particles. These carbon particles are produced due te incomplete burning of the wax vapours due to less suppy of oxygen. Carbonmonoxide is also produced along the carbon particles. This zone is luminious but with low temperature.
(iii) The inner zone:
This zone appears black in colour as no combustion of wax vapour take place in this zone due to no supply of oxygen in this zone. It is the coolest zone of the flame having unbumt wax vapour.
Combustion and Flame Class 8 HBSE Notes
1. All substances which catch fire/bum in air are called combustible substances.
2. Wood, paper, petrol, L.P.G., charcoal etc. are some examples of combustible substances.
3. During combustion heat and light is emitted. The light is emitted in the form of flame.
4. All combustible substances do not burn with flame, for example coal does not bum with flame, while wood, paper, candle, L.P.G., etc. bum with a flame.
5. There are two main things required by a substance to become combustible. These are: low ignition temperature and air.
6. Ignition temperature is the lowest temperature at which a combustible substance can easily catch fire. Inflammable substances have very low ignition temperature.
7. If any of the above condition is removed,’fire can be extinguished. Either we bring ignition temperature below minimum or we cut off the supply of air.
8. Water, sand are mostly used to bring fire under control.
9. There are three main zones of a flame. The outermost zone has the highest temperature while the inner zone has unbumt carbon particles.
10. Fuel is a substance which is used to give energy and heat. Ideal fuel has low ignition temperature and readily available. It is cheap and has high calorific value. It is free from pollution.
11. But no fuel is an ideal fuel.
12. Fuels are not same in their efficiency and cost.
13. Calorific value of fuels is expressed in units of kilojouls per kg.
14. Combustion of an inflammable substance should be complete because incomplete combustion produce unburnt carbon particle which can cause serious environmental hazards like acid rain, global warming and poisonous gases causing dangerous diseases in living beings.
15. Global warming the most alarming environmental problem is being created due to increased amount of carbondioxide in air.
16. Acidic rain is caused due to the oxides of nitrogen and sulphur released in environment due to burning of coal, diesel and petrol.