Haryana State Board HBSE 8th Class Science Solutions Chapter 14 Chemical Effects of Electric Current Textbook Exercise Questions and Answers.
Haryana Board 8th Class Science Solutions Chapter 14 Chemical Effects of Electric Current
HBSE 8th Class Science Chemical Effects of Electric Current Textbook Questions and Answers
Question 1.
Fill in the blanks:
(а) Most liquids that conduct electricity are solutions of __________ and __________.
(b) The passage of an electric current through a solution causes __________ effect.
(c) If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the __________ terfninal of the battery.
(d) The process of depositing a layer of any desired metal on another metafile object, by means of electricity, is called __________.
Answer:
(a) acids and bases
(b) chemical
(c) negative
(d) electroplating
Question 2.
When the free ends of a tester are dipped into a solution, the magnetic needle shows deflection, dan yon explain the
Answer:
Yes, the solution is a good conductor of electricity. The reason is that tote solution is a good conductor of electricity, so the needle get deflected.
Question 3.
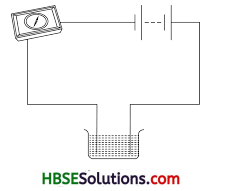
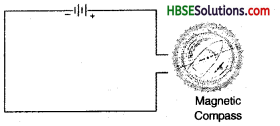
Name three liquids, which when tested in the manner shown in figure, may cause the magnetic needle to direct.
Answer:
Tap water, lime water, vinegar.
Question 4.
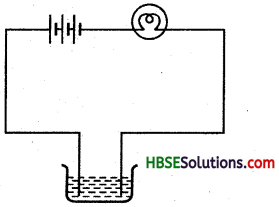
The bulb does not glow in the setup shewn in figure. List the possible reasons. Explain your answer.
Answer:
If the bulb does not glow it may be because of some reasons like the bulb may be fused. Replaced with a new bulb, if it still does not glow, it shows that the connection of wires may be loose. After tightening connetions if still the bulb does not glow, then it is for sure that the solution does not conduct electric current.
Question 5.
A tester is used to check the conduction of electricity through two liquids, labeled A and B. It is found that the bulb of the tester glows brightly for liquid A while it glows very dimly for liquid B. You would conclude that
(i) liquid A is a better conductor than liquid B.
(ii) liquid Bis a better conductor than liquid
(iii) both liquids are equally conducting.
(iv) conducting properties of liquid cannot be compared in this manner.
Answer:
(ii) liquid A is a better conductor than liquid B.
![]()
Question 6.
Does pure water conduct electricity? If not, what can we do to make it conducting?
Answer:
Some salt can be added to it to make it a conductor.
Question 7.
In case of fire, before the firemen use the water hoses, they shut off the main electrical supply for the area. Explain why they do this.
Answer:
Water is a good conductor of electric current. So, the firemen shut off the electric supply before spraying water to save themselves and other people from electrocution.
Question 8.
A child staying in the coastal region tests the drinking water and also the seawater with his tester. He finds that the compass needle deflects more in the case of seawater. Can you explain the reason?
Answer:
Drinking water has been processed and purified before supplying it to the houses. Many salts and minerals have been removed from it, so it has decreased its conductivity. On the other hand the sea water is rich in salts, and other acids and basic substances, that is why it is a better conductor of electricity and deflects the needle more than the purified drinking water.
Question 9.
Is it safe for the electrician to carry out electrical repairs outdoors during heavy downpour? Explain.
Answer:
No, it is highly dangerous to carry on electric repairs in water, as water is a good conductor of electricity. It can cause electrocution.
Question 10.
Paheli had heard that rainwater is as good as distilled water. So she collected some rainwater in a clean glass tumbler and tested it using a tester. To her surprise she found that the compass needle showed deflection. What could be the reasons?
Answer:
While it rains, the raindrops get mixed with the suspended particles of the air. So, they do not remain pure. It becomes the mixture of salts and other impurities thus shows conduction of electricity.
Question 11.
Prepare a list of objects around you that are electroplated.
Answer:
Following objects around us are electroplated, rims of cycles, door handles, taps, showers, metallic pens, artificial jewellery, utensils, metallic almirahs, buckles of clothes and belts etc.
Question 12.
The process that you saw in Activity 14.7 is used for purification of copper. A thin plate of pure copper and a thick rod of impure copper are used as electrodes. Copper from impure rod is sought to be transfered to the thin copper plate. Which electrode should be attached to the positive terminal of the battery and why?
Answer:
The impure copper rod should be attached to the positive terminal because the free copper will get drawn towards the negative terminal to be get deposted, so the copper from impure rod will get collected in the pure copper plate.
Extended Learning – Activities and Projects
Question 1.
Test the conduction of electricity through various fruits and vegetables. Display your result in a tabular form.
Answer:
For self attempt.
Question 2.
Repeat the Activity 14.7 with a zinc plate in place of the copper plate connected to the negative terminal of the battery. Now replace zinc plate with some other metallic object and again repeat the activity. Which metal gets deposited over which other metal? Discuss your findings with your friends.
Answer:
For self attempt.
Question 3.
Find out if there is a commercial electroplating unit in your town. What objects are electroplated there and for what purpose? (The process of electroplating in a commercial unit is much more complex than what we did in Activity 14.7). Find out how they dispose off the chemicals they discard.
Answer:
For self attempt.
![]()
Question 4.
Imagine that you are an ‘entrepreneur’ and have been provided a loan by a bank to set up a small electroplating unit. What object you would like to electroplate and for what purpose? (Look up the meaning of‘entrepreneur’ in a dictionary).
Answer:
For self attempt.
Question 5.
Find out the health concerns associated with chromium electroplating. How are people trying to resolve them?
Answer:
For self attempt.
Question 6.
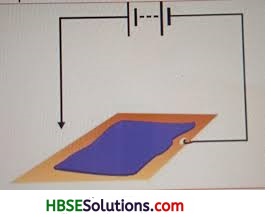
You can make a fun pen for yourself. Take a conducting metal plate and spread a moist paste of Potassium Iodide and starch. Connect the plate to a battery as shown in Fig. 14.11. Now using the free end of the wire, write a few letters on the paste. What do you see?
For more information on this topic visit:
www.tutorvista.com/content/physies/phys- ics-iv/thermalchemical-currents/chemical-ef- fects-current.php
www.physchem.co.za/Redox/ Electrolysis.htm
electronics .howstuffworks.com/3 ed ,htm
Answer:
For self attempt.
HBSE 7th Class Science Chemical Effects of Electric Current Important Questions and Answers
Very Short Answer Type Questions
Question 1.
What is electric current?
Answer:
Flow of electrons is called current.
Question 2.
How can we check current?
Answer:
By using a tester.
Question 3.
What are conductors?
Answer:
Substances which allow the electric current to pass through them are called conductors.
Question 4.
What are insulators?
Answer:
Substances which do not allow electric current to pass through them are called insulators.
Question 5.
Name any conductor.
Answer:
Copper.
Question 6.
Name any insulator.
Answer:
Plsatic.
![]()
Question 7.
Are liquids conductor of electricity?
Answer:
Yes, some liquids are conductors of electricity.
Question 8.
Name any liquid conductor.
Answer:
Tap water.
Question 9.
Can distilled water conduct electricity?
Answer:
No.
Question 10.
How can distilled water be made conductor of electricity?
Answer:
By adding some salt to it.
Question 11.
How can we check magnetic effects of electric current?
Answer:
By using magnetic compass.
Question 12.
How does magnetic compass show magnetic effect?
Answer:
By deflection.
Question 13.
What makes most of the liquids good conductors of electricity?
Answer:
Acids, bases and salts.
Question 14.
Name any two liquids except water which conduct electricity.
Answer:
Vinegar and lemon juice.
Question 15.
What is an electric circuit?
Answer:
Path of the flow of electric current is called electric circuit.
Question 16.
What in a electric circuit shows that current is passing through it?
Answer:
Glowing bulb.
Question 17.
Will a bulb glow when circuit is completed in an insulating solution?
Answer:
No.
Question 18.
Which effect of electric current causes the bulb, to glow?
Answer:
Heating effect.
![]()
Question 19.
What glows in a bulb?
Answer:
Filament
Question 20.
What is LED?
Answer:
LED is like a bulb which even glows at low or a small current.
Question 21.
What is full form of LED?
Answer:
Light Emitting Diode.
Question 22.
What are the better sources of light than bulbs and tubes?
Answer:
C.F.L.
Question 23.
What is used in CFLs?
Answer:
Mercury.
Question 24.
What other object can be used in place of bulbs to see the flow of electric current?
Answer:
Magnetic Compass.
Question 25.
What are the metal rods dipped in liquids to which cells are attached called?
Answer:
Electrodes.
Question 26.
What are the bubbles seen near electrodes after passing current through a conducting solution?
Answer:
Hydrogen and Oxygen gas.
Question 27.
When we pass current through a conducting solution of water, which gas bubbles will appear near positively charged electrode?
Answer:
Oxygen.
Question 28.
Which gas will accumulate near negative electrode when electric current is passed through a conducting solution of water?
Answer:
Hydrogen.
Question 29.
Name any chemical effect of electric current.
Answer:
Electroplating.
Question 30.
What is electroplating?
Answer:
Coating of a desired metal oft other metallic surface using electric current is Called electroplating.
![]()
Question 31.
To which terminal of the battery the metal meant for electroplating is attached?
Answer:
Negative terminal.
Question 32.
Which metal is plated on handlebars of cycles and rims of wheels?
Answer:
Chromium.
Question 33.
Which metal is plated on the iron to protect it from mist and corrosion, which is used in building bridges? .
Answer:
line.
Question 34.
Is air an insulator or conductor?
Answer:
Insulator.
Question 35.
In which direction does current flow?
Answer:
Current flows from negative terminal to the positive terminal.
Short Answer Type Questions
Question 1.
What is an electric Current?
Answer:
Plow of electrons is called electric current. Negatively charged electrons flow from negative terminal to the positive terminal and this is called electric current and the path through which it flows is called electric circuit.
Question 2.
What is a tester?
Answer:
Tester is an instrument which is Used to check the flow of electric current. It is attached to the terminal of the electric circuit, if the bulb attached to tester glows, it confirms the passage of current.
Question 3.
What are conductors?
Answer:
Substances which allow the electric current to pass through them are called conductors. Substances through which electric current passes easily are called good conductors and substances through which current passes partially or in small quantity they are called bad conductors. Substances like copper, iron, aluminium etc. are called conductors.
Question 4.
What are insulators?
Answer:
Substances which do not allow the electric current to pass through them are called insulators. Electric current do not pass through them and do not show any effect of electric current. Substances like wood, plastic, rubber* distilled water etc. are insulators.
Question 5.
Can electric current pass through liquids? Explain.
Or
Are liquids Conductor or insulator? Explain.
Answer:
Some liquids allow the electric current to pass through them and some liquids do not allow the electric current to pass through them. Mostly liquid conductors are solutions of acids, bases and salts. Liquids free from acids, bases and salts are insulators e.g. mineral water is pure form of water it is an insulator. Lemon juice vinegar are conductors.
![]()
Question 6.
You are given a solution containing lemon juice and salt.
You are told to check whether it is a conductor or insulator. How will you show it diagrammatically.
Answer:
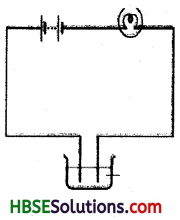
Question 7.
You are given distilled water. Check if it is a conductor or insulator?
Answer:
Distilled water is taken in a Plastic container. The free ends of the tester are dipped in the container. After waiting for a few miniutes we will find that the bulb does not glow. It confirms that distilled water does not allow electric current to pass through it so it is an insulator.
Question 8.
What is magnetic effect of current?
Answer:
When current is passed through a coil it behaves like a strong permanent magnet. The strength of the magnet depends upon the current through the wire and number of turns of the coil. This coil will continue to behave like a magnet still the current passes through it.
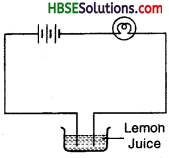
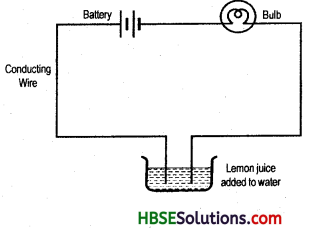
Question 9.
Draw an electric circuit showing different components like a battery, conducting wire, bulb and free ends dipped in conducting solution.
Answer:
Question 10.
What is LED, why is it a most preferable source of light?
Answer:
LED means Light Emitting Diode. Ordinary bulbs do not glow when current is small. They need large supply of current to emit light. So, they consume more current, they thus prove to be costly. On the other hand LED can glow even at very small supply of electricity and consume small current. Thus it is economical.

Question 11.
Why does a bulb glow when electric current passes through it?
Answer:
When electric current passes through a bulb, the filament of the bulb starts heating up. This is called heating effect of current. The filament gets heated up to such a high temperature that it starts glowing.
Question 12.
How can we prove conduction of substances when current is very small and the bulb does not glow?
Answer:
When current passing through a conductor is very small, the bulb does not glow. In such case we use magnetic compass to know the conduction of current. Electric current has magnetic effect which deflects the magnetic compass proving that current is flowing around the compass.
Question 13.
Why do we need magnetic compass to test the conduction of electric current?
Answer:
Sometimes when we make to flow current through a conductor, the bulb does not glow. This is because the electric current flowing through conductor is so small, that the filament of the bulb does not get heated up to the temperature where it starts glowing. So, in case of small current we need magnetic compass to test the conduction. juice is mixed in distilled water it becomes conductor of electricity.
![]()
Long Answer Type Questions
Question 1.
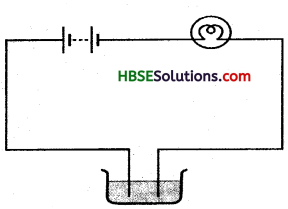
What happens when we add a pinch of salt or some lemon juice in distilled water to test its conduction of electric current?
Answer:
Distilled water does not allow the electric current to pass through it. But when we add some salt or the lemon juice to it, it starts conducting electric current and shows deflection of magnetic compass because salts and acids are conductors of electric current when salt or lemon juice is mixed in distilled water it becomes conductor of electricity.
Question 2.
Why is it not advisable to touch any electrical appliance with wet hands?
Answer:
It is not advisable to touch electrical appliances with wet hands because it can send electric shock in our body. Water is conductor of electric current. It easily allows electric current to pass through it, so when we will touch the switch or electrical appliance with wet hands the water will alow the current to pass through it and electric current will reach our body giving us shocks. This can sometimes prove very dangerous even fatal.
Question 3.
What is Electroplating? How does it take place?
Answer:
Electroplating is the process of coating a desired metal on an undesired metal surface using electric current.
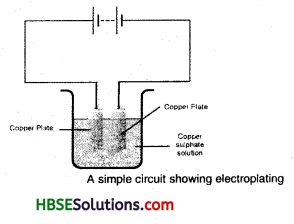
Metallic plate and the substance requiring coating are dipped in conducting solution with conducting wires. The object to be coated is attached to the negative terminal. When electric current is passed through the solution, the compounds of the conducting solution start breaking and free metallic particles get deposited on the object at negative terminal of battery. In this way we can get a coating of desired metal on any object by preparing suitable conducting solution and using suitable electrodes.
Question 4.
What are the advantages of electroplating?
Answer:
Electroplating has many advantages.
- It is used to coat metal surfaces with desired metal coatings.
- It saves metal surfaces from rusting
- It saves corrosion of surfaces of metals.
- Coating of chromium on metals give lustre to objects.
- Cheap metals like iron, aluminium etc. can be coated with costly metals like silver, gold etc. to give them rich look as in case of artificial jewellery.
- It can make reactive metals like iron etc. less reactive and they can be used for storing food items etc.
![]()
Question 5.
Show experimentally that a current carrying wire behaves like a magnet.
Answer:
As in figure shown below, a wire is fixed along the edge of a table. Place a magnetic compass near the wire. Connect the two ends of the wire to a battery cell. There is a deflection in the magnetic compass. Place the compass at different points on the table at varying distances from the wire. Observe the deflection in each case. We guess from this activity that a current carrying wire behaves like a magnet. When the current is stopped, the associated megnatic property also vanishes.
Question 6.
How can you prepare an electric pen?
Answer:
Take a filter paper and soak it into potassiumiodide and starch solution. Spread it on a metal sheet. Now join two connecting wires to the terminals of battery. Attach the wire
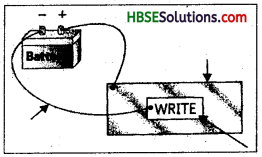
connected at positive end to plate. Now write on the sheet the wire attached to negative terminal. Wherever you will write blue coloured inck will appear. This happen due to electrolysis of potassium iodide solution which produces iodine. Iodine on reaction with starch produce ink of blue colour.
Chemical Effects of Electric Current Class 8 HBSE Notes
- Flow of electrons is called current.
- Those objects which allow the current to pass though them are called conductors.
- Those objects which do not allow the current to pass through them are called insulators.
- Objects like copper, aluminium etc. and almost all metals are good conductors of electricity.
- Objects like wood, plastic, rubber etc. do not allow electricity to pass through totem and are – thus bad conductors of electricity and are thus called insulators.
- liquids like’vinegar, lemon juice, tap water etc. are conductors of electricity and distilled water is an insulator. Salted distilled water becomes a conductor.
- Electric current has many chemical effects.
- Electric current when passed through a conducting solution causes different changes in it, these are called chemical changes.
- It may evolve gas after breaking the chemical solution into different elements. Chemical effects depend upon the conducting solution and the electrodes used.
- Electrodes are the rods of different metals immersed in conducting solution to complete the circuit and cause flow of electrons.
- Electric current when passed through a conducting solution can cause electroplating.
- Electroplating is the process of depositing layer of any suitable metal on another metal.