Haryana State Board HBSE 10th Class Science Notes Chapter 1 Chemical Reactions and Equations Notes.
Haryana Board 10th Class Science Notes Chapter 1 Chemical Reactions and Equations
Reaction: Reactions are of two types. They are:
Physical reaction (Physical change)
It is a change in the physical properties of a substance.
For e.g., melting, heating, dissolving, etc.
Chemical reaction (Chemical change)
One or more reactants (substances) getting chemically changed into one or more new products is called chemical reaction.
Components of a chemical reaction:
- Reactants: Substances that undergo reaction.
- Products: Substances produced when reactants undergo reaction.
- Chemical equation: The method of representing a chemical reaction using symbols and formulae of substances involved (Le. reactants and products) is known as a chemical equation.
![]()
Types of chemical reaction:
Combination reaction: Two or more reactants combine to form a single product.
E.g.: CaO + H2O → Ca(OH)2
Decomposition reaction: A single reactant breaks down to give two or more simpler products. (Decomposition reaction can be considered as opposite of combination reaction.)
E.g.:

To decompose a reactant one needs to provide heat, electric current, light, etc. Based on this the types of decompositión reaction are —
- Thermal decomposition
- Electrical decomposition
- Light decomposition
![]()
Displacement reaction: A reaction in which a more reactive element displaces i.e. removes less reactive element from its compound.
Types:
(a) Single displacement reaction (or simply displacement reaction):
E.g.: Fe + CuSO4 → FeSO + Cu
Here, iron (Fe) removes (displaces) copper (Cu) from its compound CuSO4.
In this reaction, the more reactive element reacts with the compound of less reactive element and displaces or say takes the place of the less reactive element in that compound.
(b) Double-displacement reaction:
- It is the reaction in which two different ions or group of atoms in the reactant molecules are displaced by each other.
- Precipitates are produced in this reaction and so it is also called precipitation reaction.
E.g.: Na2SO4 + BaCl2 → BaSO4 + 2NaCl
Oxidation and reduction reactions:
(a) Oxidation: It is the reation in which (a) either oxygen (O2) is added to a substance or (b) Hydrogen (H2) is removed from a substance.
E.g.:
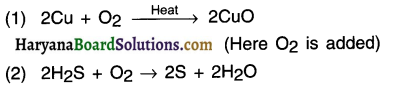
(b) Reduction: It is the opposite of oxidation. In this,
(a) Either oxygen is removed from a substance or
(b) Hydrogen is added to a substance.
E.g.:
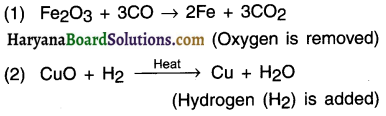
(c) Redox reaction: It is a reaction in which oxidation and reduction takes place simultaneously. (In Redox, Red = reduction, Ox = oxidation) E.g.
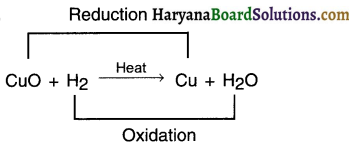
CuO got reduced to Cu (since oxygen is removed).
This is reduction reaction. H2 gets oxidized via. oxidation to form H2O. This is oxidation reaction.
![]()
Exothermic and endothermic reactions: In a chemical reaction, if heat is evolved/released it is called exothermic (combustion) reaction. E.g. burning of natural gas:
CH4 + 2O2 → CO2 + 2H2O + Heat
Endothermic reaction: A reaction in which heat is absorbed or say required is called endothermic reaction.
Example:
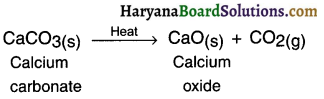
When calcium carbonate is supplied heat, calcium oxide and carbon dioxide are formed.
Effect of Oxidation reaction in everyday life:
Corrosion:
- When a metal comes in contact with humid air, moisture or a chemical such as acid, the surface of metal starts getting eaten up. This is called corrosion.
- Corrosion is mainly caused by the oxidation of metals in humid air. Rusting of iron is the most common form of corrosion.
![]()
Rancidity:
- Oxidation affects food that contains fats and oils.
- When food items (such as snacks like pun, chakri, chavana, etc.) prepared using fat and oils are kept for longer period, they develop an unpleasant smell and taste. We then say that the food item has become rancid.